An alkaline aqueous sodium ion battery
An alkaline aqueous solution, sodium-ion battery technology, applied in the direction of alkaline storage battery, alkaline storage battery manufacturing, alkaline electrolyte, etc., can solve problems such as increasing cost, achieve high conductivity and stability, cheap raw materials, good commercial The effect of changing the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
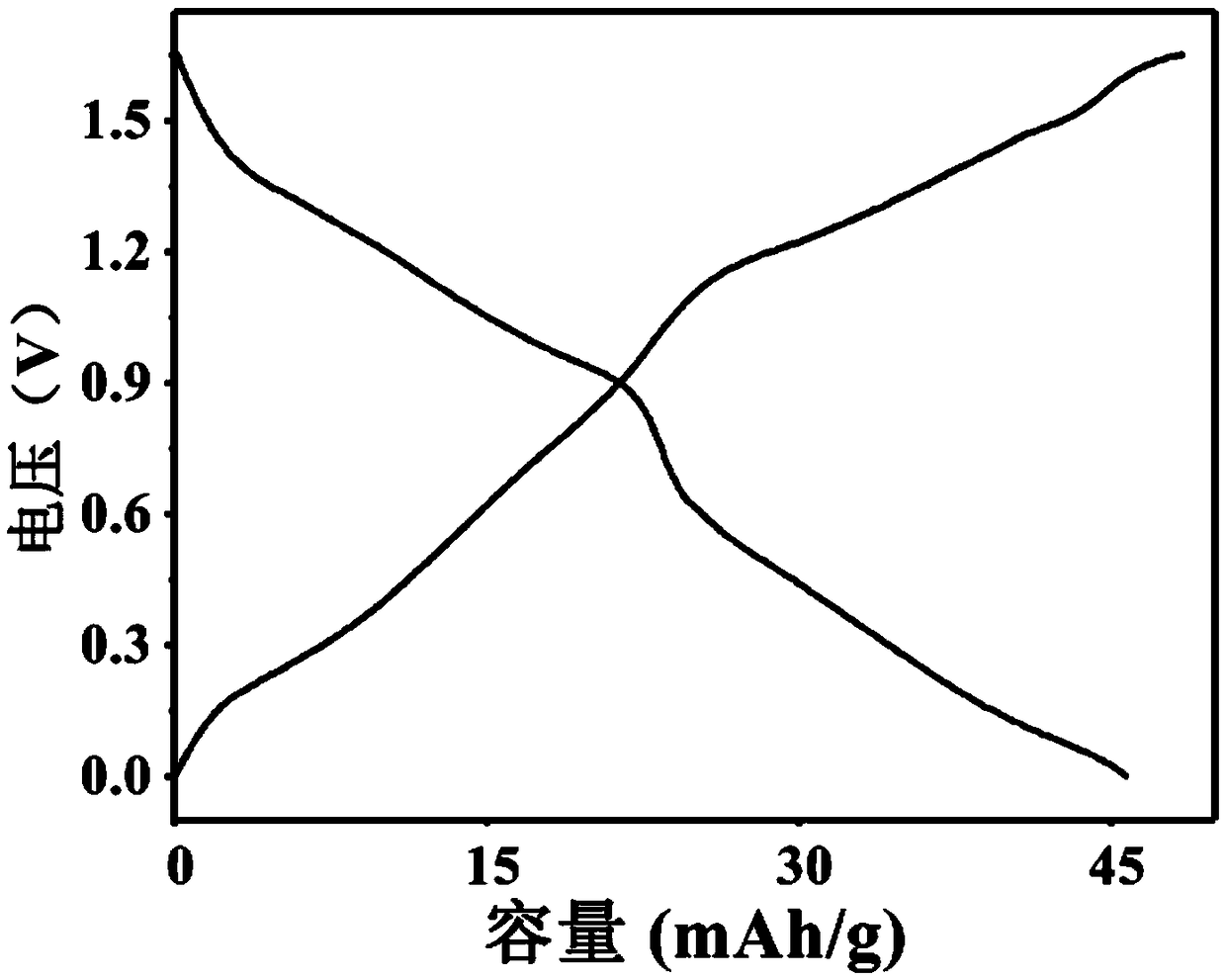
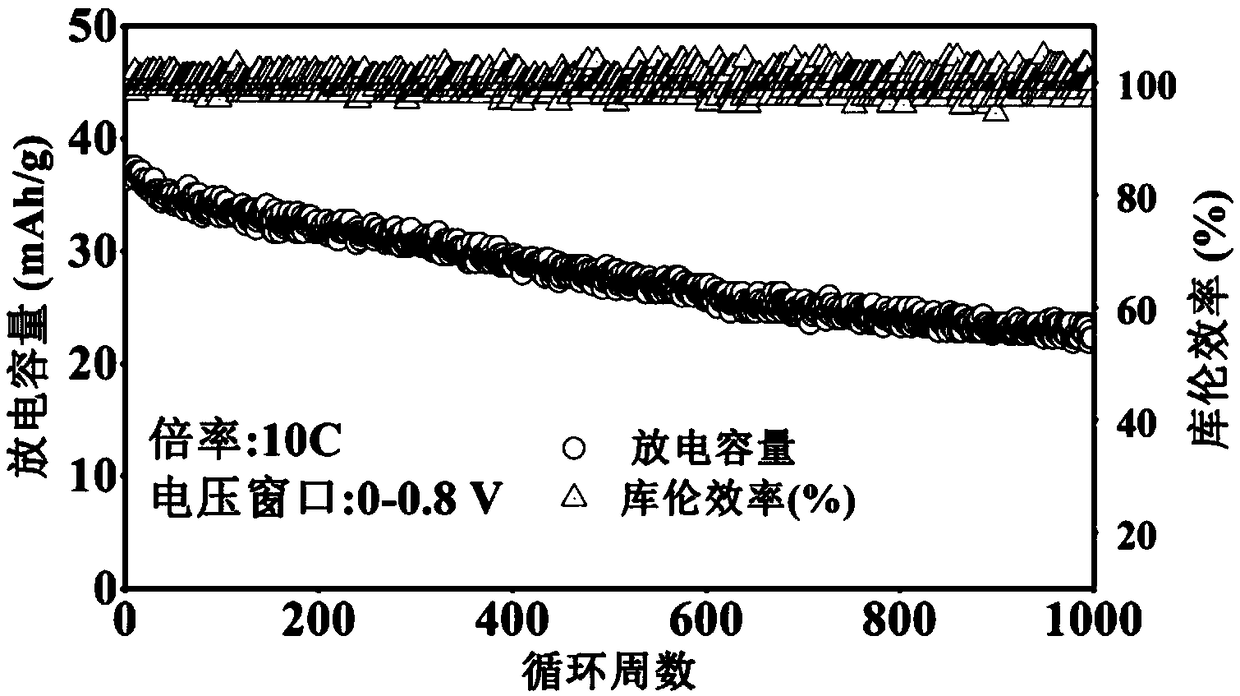
[0055] This example describes a tunnel oxide Na 0.5 mn 0.9 Fe 0.1 o 2 It is an alkaline aqueous sodium ion battery with a zinc sheet as the positive electrode and the negative electrode.
[0056] (1) Positive part: Na 0.5 mn 0.9 Fe 0.1 o 2 Synthesis: Dissolve sodium acetate, manganese acetate tetrahydrate and ferric citrate in absolute ethanol at a molar ratio of 0.5:0.9:0.1, heat and stir at 50-90°C in a heat-collecting magnetic stirrer, and slowly add deionized water until all salts are completely dissolved. Then add phenol and formaldehyde solution to the mixed solution. Continue to heat and stir until the mixed solution becomes a gel-like solid. Transfer the gel-like solid into a vacuum oven at 50-100° C., and dry it in vacuum for 8-15 hours. The dried solid is fully ground, put into a muffle furnace under an air atmosphere, and calcined at 700-900° C. for 8-15 hours.
[0057] Na 0.5 mn 0.9 Fe 0.1 o 2 Electrode preparation: Na 0.5 mn 0.9 Fe 0.1 o 2 It is...
Embodiment 2
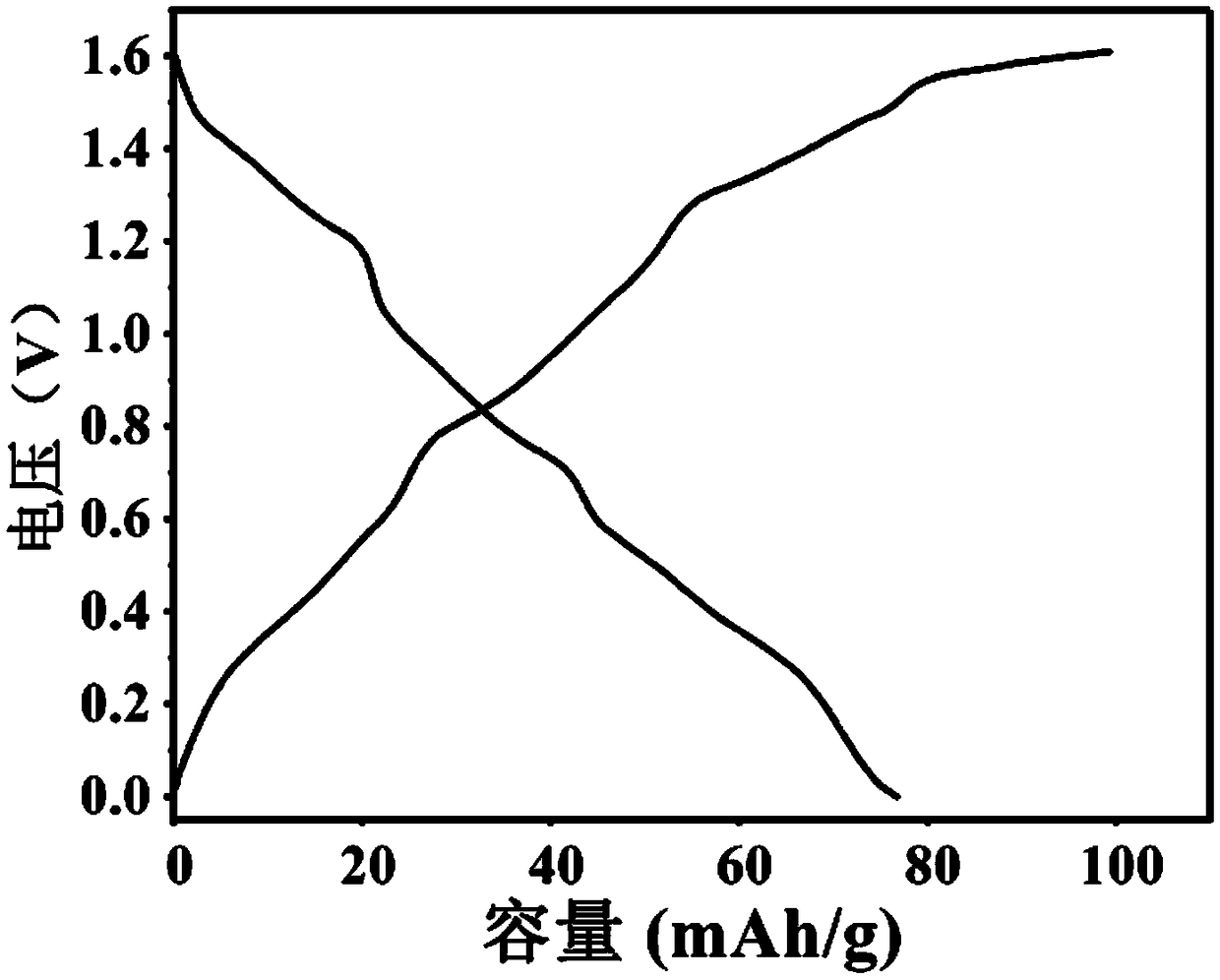
[0062] This example describes a tunnel oxide Na 0.5 mn 0.9 Al 0.1 o 2 It is an alkaline aqueous sodium ion battery with activated carbon as the positive electrode and the negative electrode.
[0063] (1) Positive part: Na 0.5 mn 0.9 Al 0.1 o 2 : The molar ratio is 0.5:0.9:0.1 Weigh sodium acetate, manganese acetate tetrahydrate and aluminum nitrate and dissolve in absolute ethanol, heat and stir at 50-90°C in a collector-type magnetic stirrer, and slowly add deionized water until all salts are completely dissolved. Then add phenol and formaldehyde solution to the mixed solution. Continue to heat and stir until the mixed solution becomes a gel-like solid. Transfer the gel-like solid into a vacuum oven at 50-100° C., and dry it in vacuum for 8-15 hours. The dried solid is fully ground, put into a muffle furnace under an air atmosphere, and calcined at 700-900° C. for 8-15 hours.
[0064] Na 0.5 mn 0.9 Al 0.1 o 2 Electrode preparation: Na 0.5 mn 0.9 Al 0.1 o 2 ...
Embodiment 3
[0069] This example describes a tunnel oxide Na 0.5 mn 0.9 co 0.1 o 2 It is an alkaline aqueous sodium ion battery with a zinc sheet as the positive electrode and the negative electrode.
[0070] (1) Positive part: Na 0.5 mn 0.9 co 0.1 o 2 Synthesis: Weigh sodium acetate, manganese acetate tetrahydrate and cobalt nitrate at a molar ratio of 0.5:0.9:0.1 and dissolve them in absolute ethanol, heat and stir at 50-90°C in a heat-collecting magnetic stirrer, while slowly adding deionized water until all salts are completely dissolved. Then add phenol and formaldehyde solution to the mixed solution. Continue to heat and stir until the mixed solution becomes a gel-like solid. Transfer the gel-like solid into a vacuum oven at 50-100° C., and dry it in vacuum for 8-15 hours. The dried solid is fully ground, put into a muffle furnace under an air atmosphere, and calcined at 700-900° C. for 8-15 hours.
[0071] Na 0.5 mn 0.9 co 0.1 o 2 Electrode preparation: Na 0.5 mn 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com