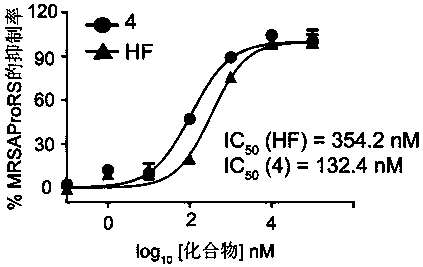
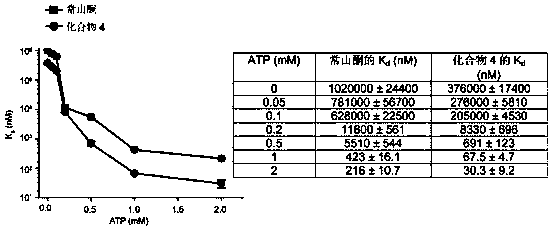
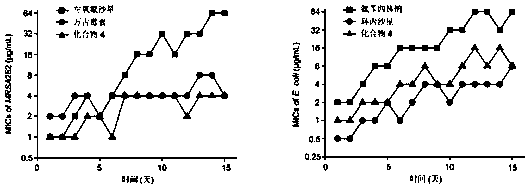Deoxidized halofuginone compound and preparation method and application thereof
A technology for halofuginone and compound, which is applied in the field of deoxyhalofuginone compound and its preparation, can solve the problem of not finding a targeted bacterial type ProRS and the like, and achieves the effects of good development prospects, cheap and easy-to-obtain raw materials, and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of (R)-3-butyl-2-(3-bromo-2-propionyl)piperidine-1-carboxylate (compound 23)
[0034] Piperidine (100mmol), NCS (111.6mmol) was rapidly stirred in ether (500mL), and reacted at room temperature for 4h. After the reaction was complete, the residue was removed by filtration, and the filtrate was concentrated at room temperature to about 80 mL, then added dropwise to potassium hydroxide (100 mmol) in ethanol (50 mL) in an ice bath, reacted overnight, and filtered to obtain filtrate A. Sodium hydroxide (150mmol) and ethyl acetoacetate (100mmol) were dissolved in solvent water, heated at 50°C for 4h, and stirred overnight at room temperature to obtain B. A and B were mixed and refluxed for 4h to obtain a yellow solution. It was extracted with dichloromethane (3×75 mL), the organic phase was dried over anhydrous magnesium sulfate, and the organic solvent was removed under reduced pressure to obtain a crude product. The crude product was stirred with di...
Embodiment 2
[0035] The preparation of embodiment 2 deoxyhemoshenone compound (compound 1-15)
[0036] Compound 23 (2mmol), the corresponding substituted quinazolinone (2mmol), potassium carbonate (6mmol) were reacted in the solvent DMF (5mL) for 2-8h. The reaction was detected by TLC. After the reaction was complete, excess water was added, and a solid was precipitated at room temperature. Intermediate D was obtained by suction filtration. Intermediate D was dissolved in 1,4-dioxane (3 mL), and concentrated hydrochloric acid was added dropwise at room temperature for 4-6 days. Detected by TLC, after the reaction was complete, the organic solvent was removed under reduced pressure. The mobile phase (dichloromethane:methanol (v / v)=1:7) was passed through a silica gel column to obtain compound 1-15 with a yield of about 75-90%.
Embodiment 3
[0037] The preparation of embodiment 3 deoxyhemoshenone compound (compound 16)
[0038]Compound 2 (2mmol) and sodium borohydride (2mmol) were reacted in methanol (2mL) on ice for 1h. The progress of the reaction was detected by TLC. After the reaction was complete, the organic solvent was removed under reduced pressure. The mobile phase (dichloromethane: methanol (v / v) = 1:6) was passed through a silica gel column to obtain compound 16. Its yield is about 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com