Method for removing gaseous mercury in flue gas through catalytic oxidation of cobalt sulfide/biomass carbon composite material
A technology of biomass carbon and composite materials, applied in chemical instruments and methods, physical/chemical process catalysts, separation methods, etc., can solve the problems affecting the oxidation and removal of elemental mercury, no catalytic oxidation and mercury removal catalysts, and HCl dependence. Low problems, to achieve the effect of improving flue gas treatment capacity, mild conditions, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
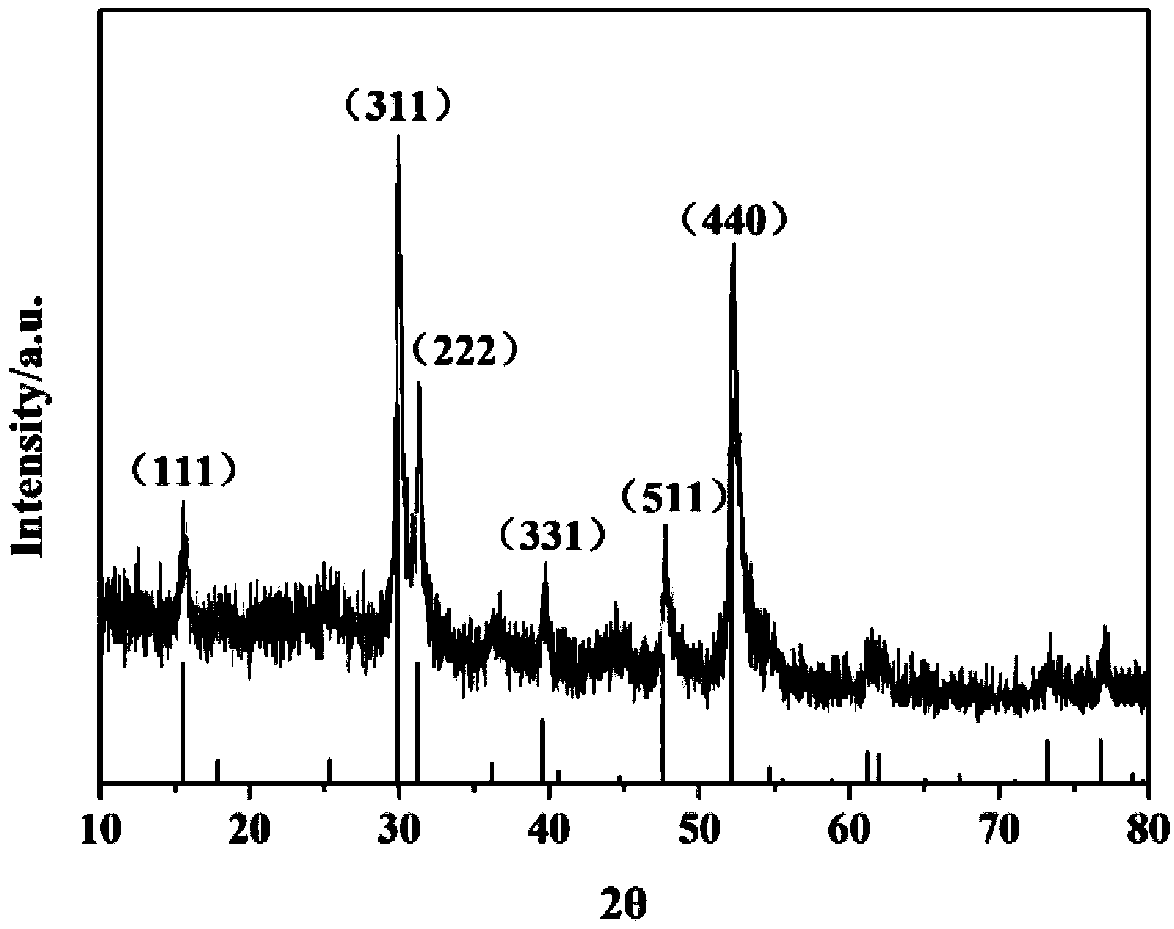
[0046] Will analyze pure cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O) Add to deionized water to make Co 2+ A red solution with a concentration of 0.1 mol / L, the resulting solution is marked as A; add analytically pure thiourea (SC(NH 2 ) 2 ), make Co in the mixed solution 2+ With SC(NH 2 ) 2 The molar concentration ratio of is 0.5:1, stirring for 3h, the resulting solution is denoted as B; 3g of biomass (grapefruit peel) is added to solution B for mixing and dipping, standing for 24h, drying in 80℃ oven to obtain sample C; C was placed in a tube furnace at 800°C and fired in a nitrogen atmosphere for 2 hours at a heating rate of 5°C / min; after firing, it was naturally cooled to room temperature in a nitrogen atmosphere and taken out of the firing tube to obtain black cobalt sulfide / Biomass carbon composite material. The sample obtained was analyzed by the Rigaku D / Max 2500VB+XX X-ray diffractometer, and it was found that the crystal phase of the obtained product was cobalt-nickel-pyrit...
Embodiment 2
[0048] Catalyst oxidation activity and test of the influence of different concentrations of HCl on catalyst activity:
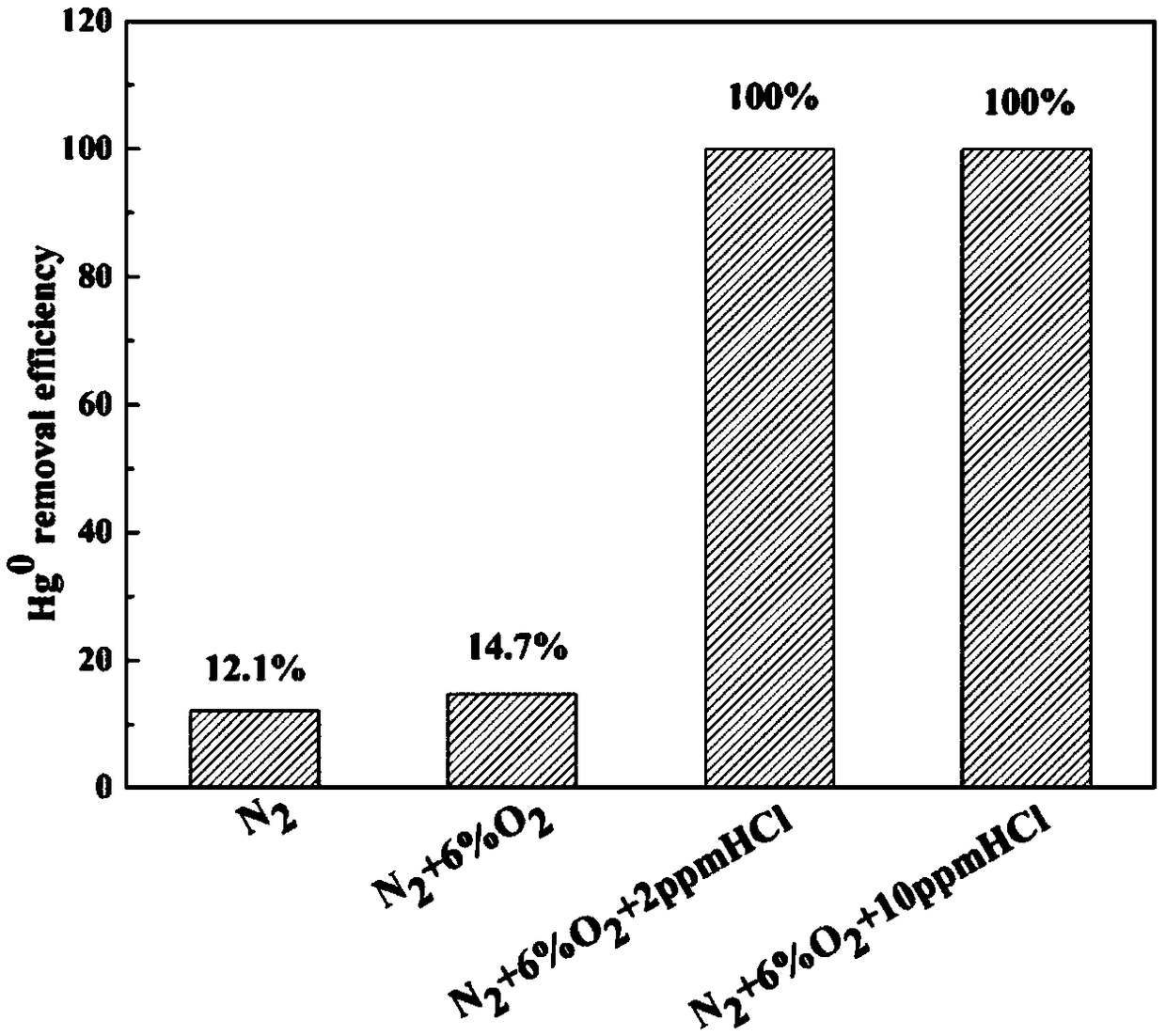
[0049] The experiment was carried out in a fixed bed reactor, the catalyst (prepared in Example 1) was filled with 50 mg, and N 2 As Hg 0 Carrier gas, control its flow to 200mL / min, Hg in the flue gas 0 The concentration is 260μg / m 3 Left and right, another way N 2 As the balance gas, the total flow of the gas is maintained at 600 mL / min, and the experimental temperature is 150°C. Set the experimental atmosphere conditions, reaction 1: do not add any oxidizing gas, only pass in nitrogen as carrier gas and balance gas; reaction 2: pass in 6% O 2 , The rest of the gas is N 2 ; Reaction 3: pass 6% O 2 , 2ppm HCl, the remaining gas is N 2 ; Reaction 4: pass 6% O 2 , 10ppmHCl, the rest of the gas is N 2 . After flue gas treatment, the catalyst treatment efficiency was recorded after the mercury concentration reached a stable level. The result is image 3 Shown.
Embodiment 3
[0051] Test of temperature influence on catalyst activity:
[0052] The experiment was carried out in a fixed bed reactor, the catalyst (prepared in Example 1) was filled with 50 mg, and N 2 As Hg 0 Carrier gas, control its flow to 200mL / min, Hg in the flue gas 0 The concentration is 260μg / m 3 Left and right, another way N 2 As a balance gas, keep the total flow of gas at 600mL / min, and pass in an oxidizing atmosphere of 6% O 2 And 10ppm HCl. Set the reaction temperature conditions, reaction 1: 50°C; reaction 2: 100°C; reaction 3: 150°C; reaction 4: 200°C. After flue gas treatment, the catalyst treatment efficiency was recorded after the mercury concentration reached a stable level. The result is Figure 4 Shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com