Method for preparing AlN powder
A powder and solid technology, applied in chemical instruments and methods, nanotechnology for materials and surface science, inorganic chemistry, etc., can solve the problems of long reaction time, high synthesis temperature, complex process, etc., and achieve simple preparation method , low reaction temperature, environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] According to the Al:N molar ratio of 1:18, weigh 2.4143g (0.01mol) AlCl 3 ·6H 2 O and 5.7318 g (0.06 mol) of guanidine hydrochloride were fully dissolved in 15 mL of ethanol, and then the ethanol was evaporated to dryness at 80° C., and the resulting solid mixture was calcined at 800° C. for 2 h in a nitrogen atmosphere, and then decarburized at 550° C. for 3 h in an air atmosphere. Cool naturally to room temperature to obtain AlN powder.
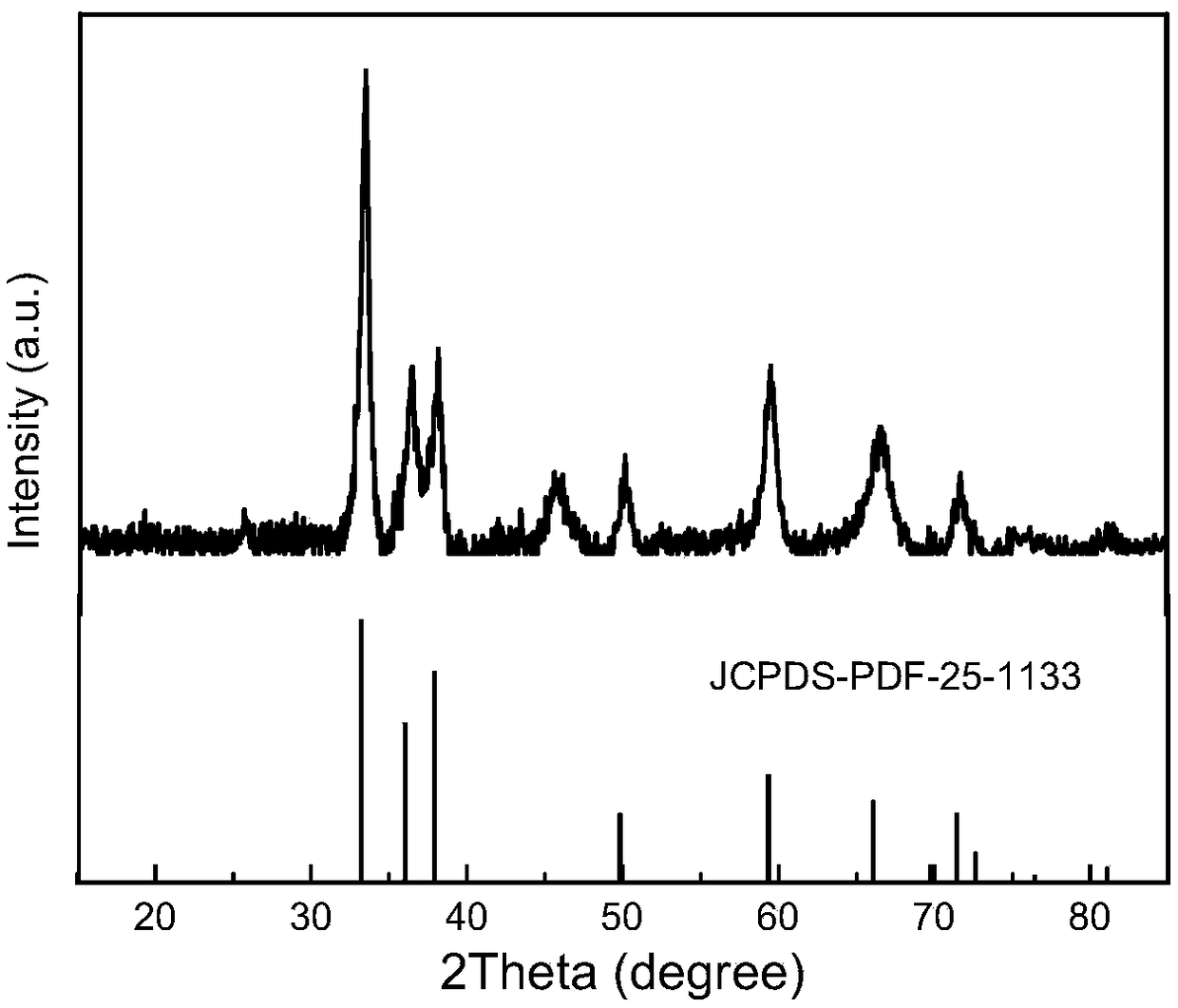
[0031] Depend on figure 1 It can be seen that the XRD diffraction peaks of the obtained AlN powder are consistent with the standard diffraction peaks, indicating that the prepared AlN has high purity. At the position of the diffraction peak with a 2θ value of 33°, the size of the AlN crystal is calculated using the Debye-Scherrer formula to be 9.4 nm, which means the average particle size of the crystal in the vertical direction of 33°.
Embodiment 2
[0033] According to the Al:N molar ratio of 1:24, weigh 2.4143g (0.01mol) AlCl 3 ·6H 2 O and 7.6424 g (0.08 mol) of guanidine hydrochloride were fully dissolved in 15 mL of methanol, and then the methanol was evaporated to dryness at 70° C., and the resulting solid mixture was roasted at 800° C. for 2.5 h in a nitrogen atmosphere, and then decarburized at 550° C. for 1 h in an air atmosphere. Cool naturally to room temperature to obtain AlN powder with a crystal size of 8.6 nm.
Embodiment 3
[0035] According to the Al:N molar ratio of 1:15, weigh 2.4143g (0.01mol) AlCl 3 ·6H 2 O and 5.8555g (0.05mol) of guanidine acetate were fully dissolved in 15mL of deionized water, then evaporated to dryness at 100°C, and the resulting solid mixture was placed in a mixed atmosphere of ammonia and helium at a volume ratio of 1:9 at 850°C Calcined for 1 hour, then decarburized at 550°C for 3 hours in an air atmosphere, and cooled naturally to room temperature to obtain AlN powder with a crystal size of 15.9 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com