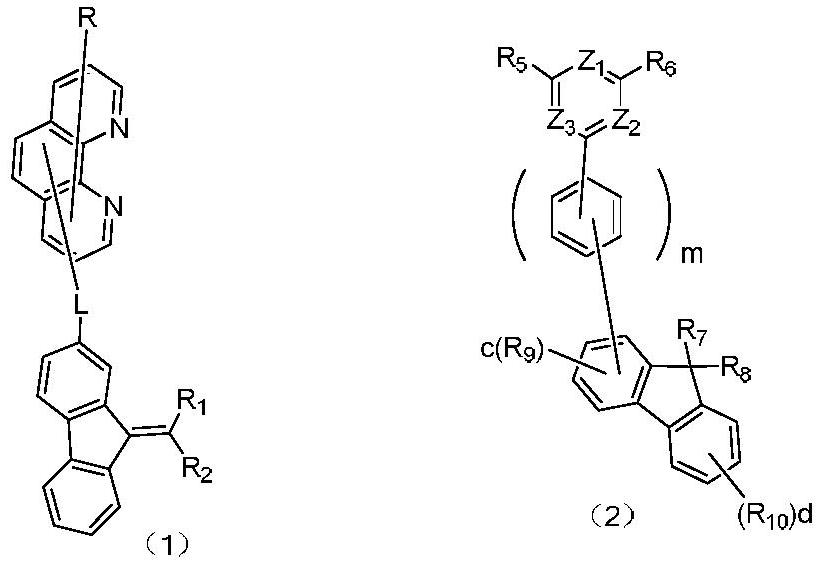
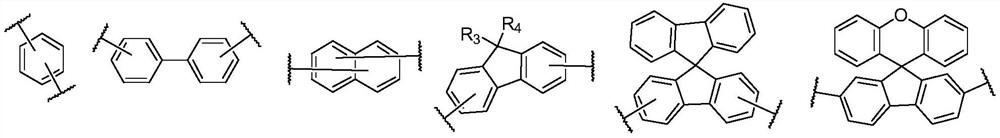
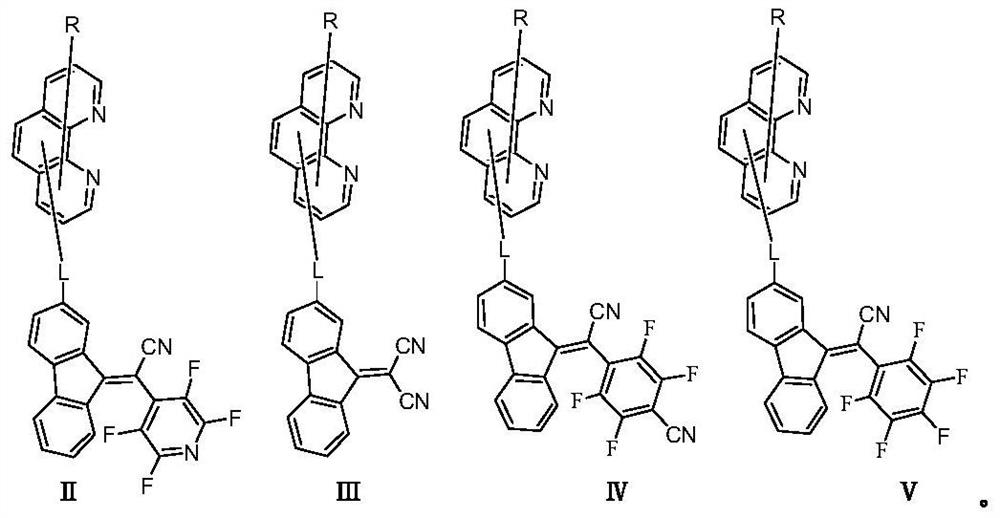
An organic light emitting device
A technology of organic light-emitting devices and light-emitting layers, which is applied in the manufacture of semiconductor devices, electric solid-state devices, and semiconductor/solid-state devices. rate, to avoid unbalanced effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0100] The preparation of organic light-emitting devices can be any of vacuum evaporation, spin coating, vapor deposition, blade coating, laser thermal transfer, electrospray coating, slit coating, and dip coating. One, the vacuum evaporation method is preferably used in the present invention.
[0101] The organic light-emitting device of the present invention can be widely used in the fields of panel display, lighting source, flexible OLED, electronic paper, organic photoreceptor or organic thin film transistor, signboard, signal lamp and the like.
Embodiment 1
[0104] [Example 1] Synthesis of Compound 1-3
[0105]
[0106] Step1: Add compound 1-3-e (12.71g, 37.6mmol) to the reactor and dissolve in tetrahydrofuran (140mL), add dropwise hexane solvent and 2.5M n-butyllithium (18mL, 45.1mmol) at -78°C ), stirred for 1 hour. After slowly adding trimethyl borate (13 mL, 56.4 mmol) dropwise, stirred for 2 h. Then 2M hydrochloric acid was added dropwise to neutralize, and the product was extracted with ethyl acetate and water. Recrystallization from dichloromethane and hexane gave compound 1-3-A (4.73 g, 47%).
[0107] Step2: Add compound 1-3-A (28.81g, 100mmol), compound 1-3-g (12.30g, 100mmol), tetrakistriphenylphosphine palladium (1.15g, 1mmol) and sodium carbonate (41.4g) in the reactor , 300 mmol), the weighed reactant was dissolved in a solvent of toluene (1 L) / EtOH (200 mL) / distilled water (200 mL), and heated at 90° C. for 2 hours. The reaction mixture was cooled to room temperature, diluted with toluene and filtered through ...
Embodiment 2
[0111] [Example 2] Synthesis of Compound 1-5
[0112] Compound 1-3-g in compound 1-3 in Example 1 was replaced by equimolar compound 1-5-g, and compound 1-5 (36.57 g, 58%) was obtained according to the synthesis method of compound 1-3.
[0113]
[0114] Mass Spectrum m / z: 630.16 (calculated: 630.15). Theoretical element content (%)C 40 h 18 f 4 N 4 : C, 76.19; H, 2.88; F, 12.05; N, 8.88 The measured element content (%): C, 76.19; H, 2.88; F, 12.06; N, 8.87. The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com