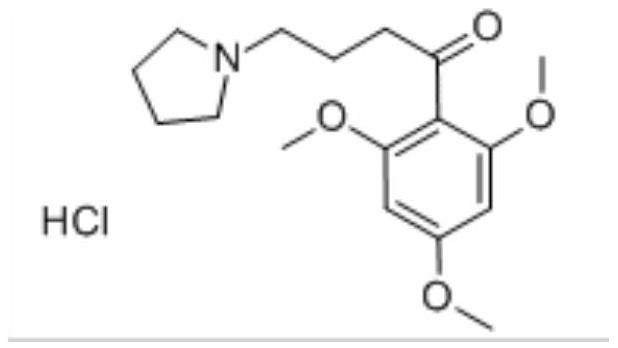
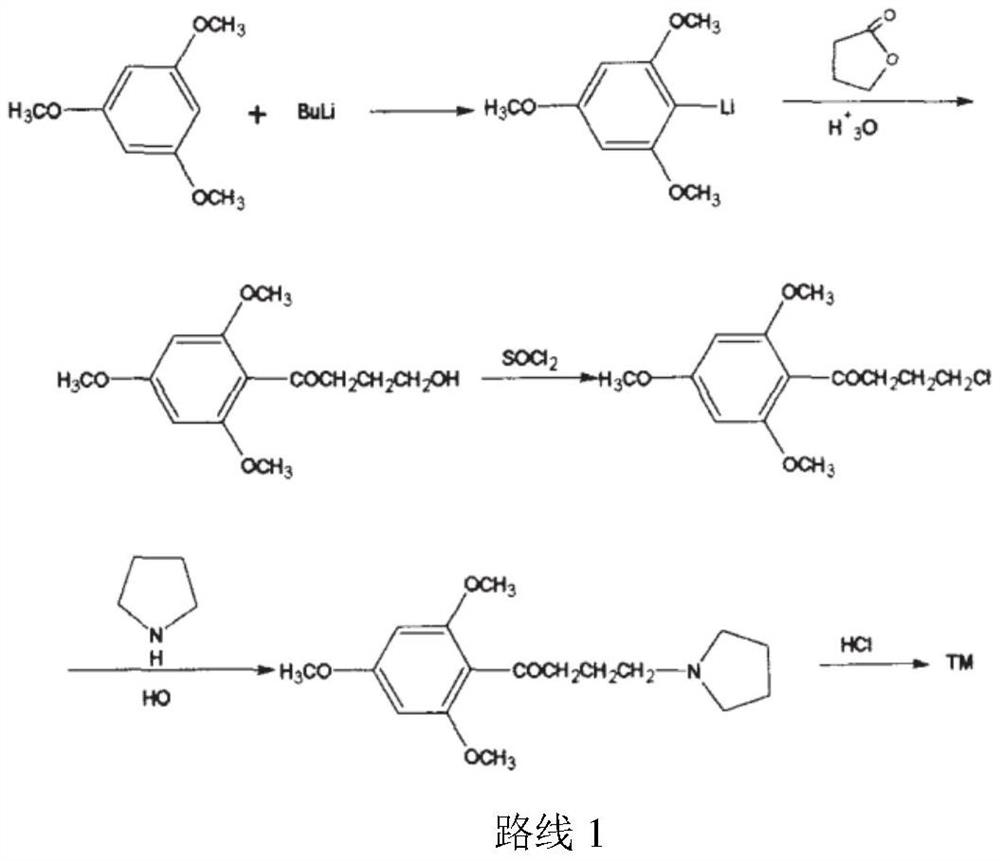
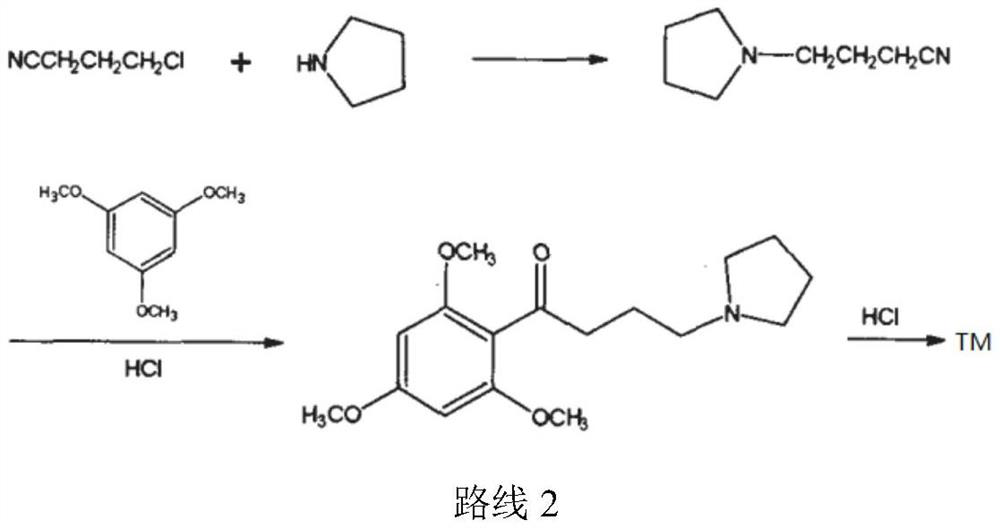
The synthetic method of buflomedil hydrochloride intermediate 1,3,5-trimethoxybenzene
A technology of buflomedil hydrochloride and trimethoxybenzene, which is applied in the field of synthesis of buflomedil hydrochloride intermediate 1,3,5-trimethoxybenzene, can solve the problem of low yield, unfavorable safe operation, unfavorable safety Use and other problems to achieve the effect of high reaction yield, simple post-treatment operation, and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthetic method of 1,3,5-trimethoxybenzene comprises the following steps:
[0035] 1) Mix methanol and catalyst evenly, pass in argon, control the pressure to 7 atmospheres, control the temperature to 135°C, keep it for 30 minutes, add dropwise a solution composed of 1,3,5-tribromobenzene and toluene, and control the The dripping time of the solution is 45 minutes. After the solution is added, triethylamine is started to be added dropwise. The dripping time of triethylamine is controlled to be 10 minutes. , and then continue to react for 11h and the reaction ends.
[0036] The preparation method of the catalyst is as follows: mix and grind sodium oxide and barium oxide, pass through a 700-mesh sieve, take the under-sieve to activate at 800°C, mix and grind the obtained mixture with dextran gel, and pass through a 500-mesh sieve to obtain; The weight ratio of sodium to barium oxide is 1:0.32; the weight ratio of the activated mixture to Sephadex is 1:55; the Sephad...
Embodiment 2
[0040] The synthetic method of 1,3,5-trimethoxybenzene comprises the following steps:
[0041] 1) Mix methanol and catalyst evenly, feed nitrogen, control the pressure to 5 atmospheres, control the temperature to 120°C, keep it for 20 minutes, add dropwise a solution composed of 1,3,5-trichlorobenzene and benzene, and control the solution dropwise Adding time is 35 minutes. After adding the solution, start to add pyridine dropwise. Control the dropping time of pyridine to be 5 minutes. After adding pyridine, raise the temperature to 155° C., raise the pressure to 9 atmospheres, and then continue the reaction for 7 hours to complete the reaction.
[0042] The preparation method of the catalyst is: mix and grind sodium oxide and barium oxide, pass through a 500 mesh, take the under-sieve and activate at 700°C, then mix and grind the activated mixture with dextran gel, pass through a 400 mesh sieve to obtain The weight ratio of sodium oxide to barium oxide is 1:0.25; the weight r...
Embodiment 3
[0046] The synthetic method of 1,3,5-trimethoxybenzene comprises the following steps:
[0047] 1) Mix methanol and catalyst evenly, pass in argon, control the pressure to 8 atmospheres, control the temperature to 145°C, keep it for 35min, add dropwise a solution composed of 1,3,5-triiodobenzene and xylene, and control The solution dripping time is 50min. After the solution is added, N,N-diisopropylethylamine is added dropwise, and the dripping time of N,N-diisopropylethylamine is controlled to be 12min. After the addition of ethylethylamine, the temperature was raised to 170°C, the pressure was raised to 12 atmospheres, and the reaction was continued for 13 hours to complete the reaction.
[0048] The preparation method of the catalyst is as follows: mix sodium oxide and barium oxide, grind them, pass through 800 meshes, take the undersieve and activate them at 850°C, then mix and grind the activated mixture with dextran gel, pass through a 600 meshes sieve to obtain The weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com