Method for preparing ultra-low-molecular-weight keratin peptide, and use thereof
A technology of keratin peptide and keratin, which is applied in the field of preparation and utilization of ultra-low molecular keratin peptide, can solve the problems of low economy and productivity, achieve excellent skin keratin permeability, prevent skin aging or skin The effect of wrinkles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 . Preparation of Keratin Peptides
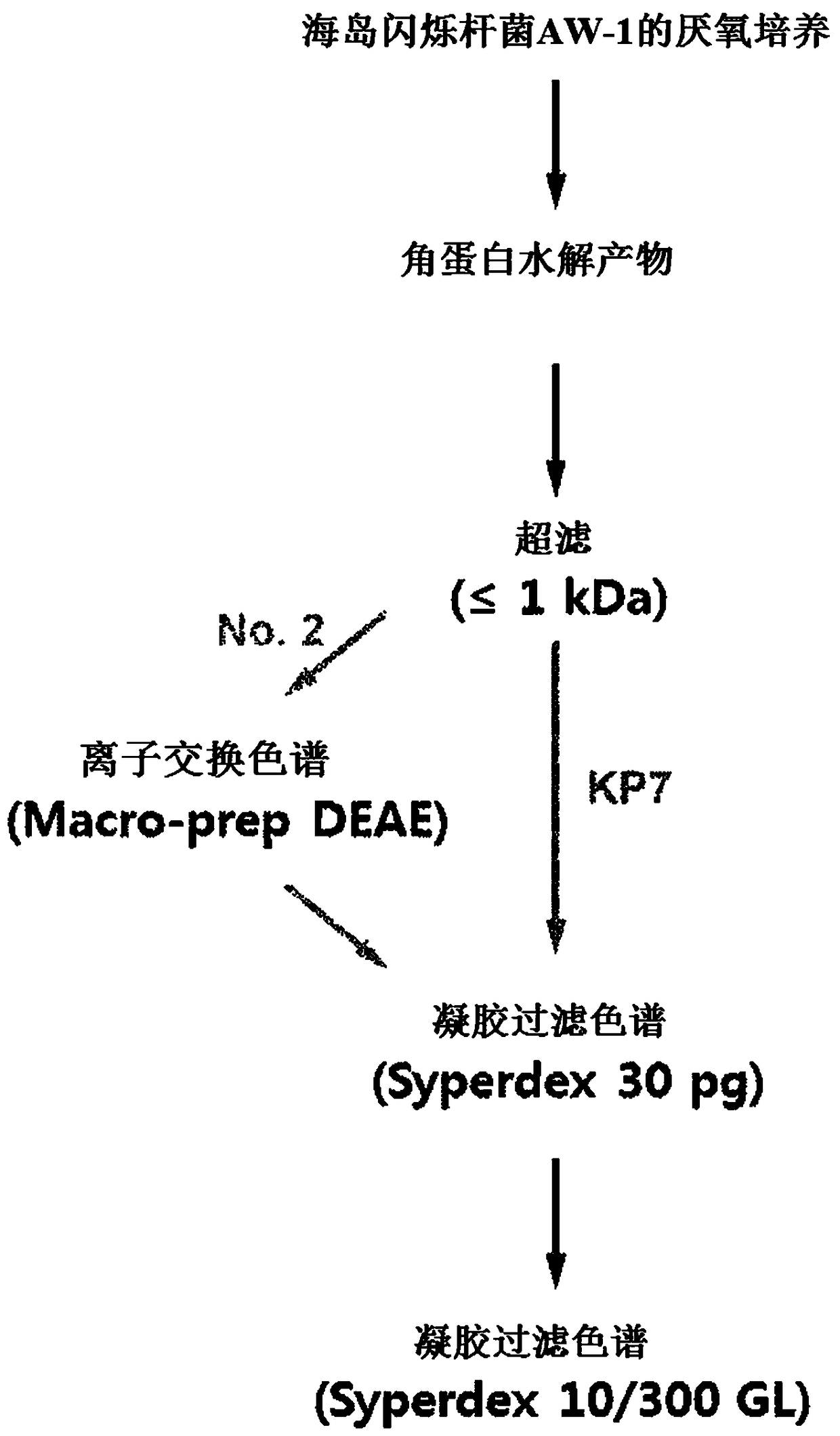
[0077] 1.1 Culture and preparation of keratin hydrolyzate
[0078] In order to prepare the keratin peptide mixture, as shown in the following table 1, using a nitrogen source, under the temperature condition of 70 ℃, in the growth medium supplemented with chicken feathers, Fervidobacterium islandicum (Fervidobacterium islandicum) AW-1 (Fervidobacterium islandicum) AW-1 ( KCTC4680) were anaerobically cultured to obtain keratin hydrolyzate.
[0079] Table 1
[0080] .
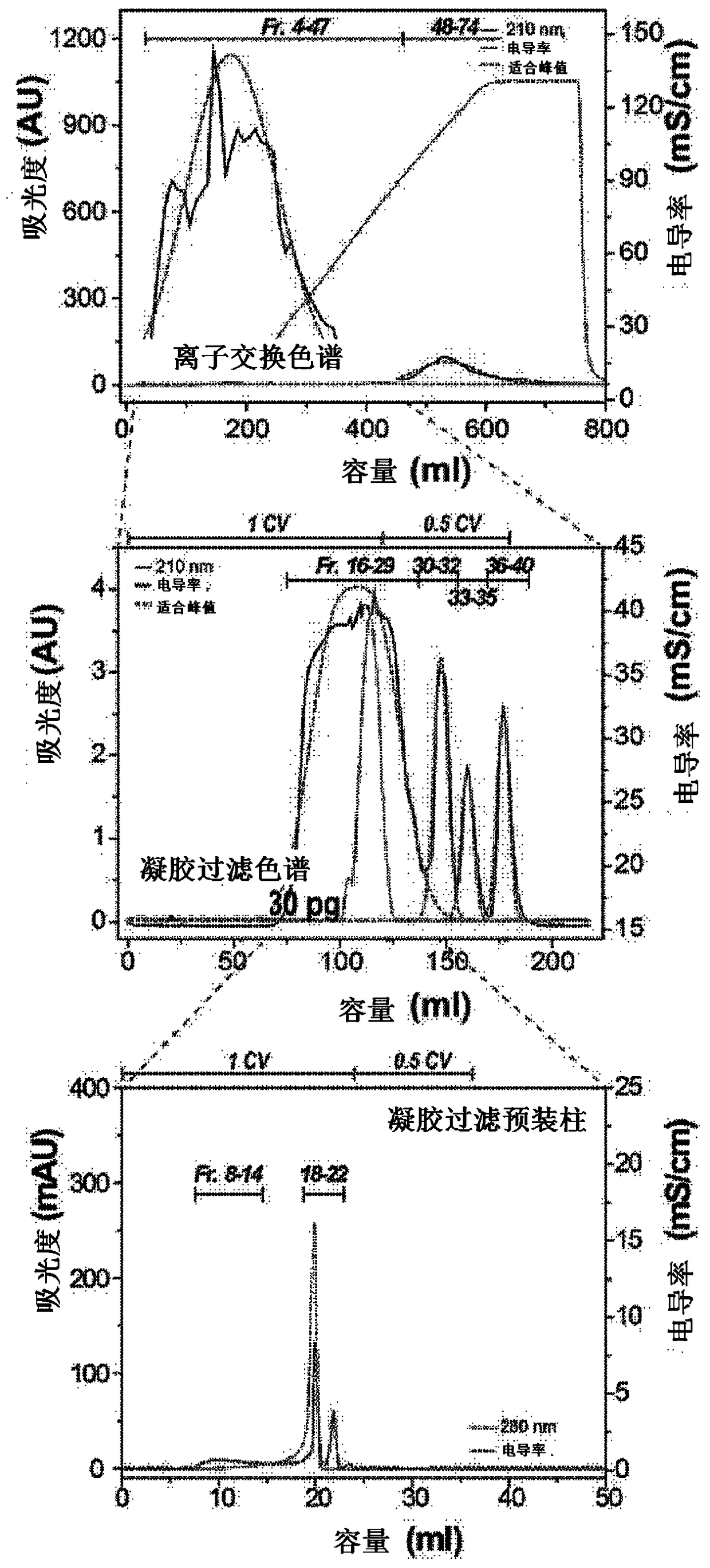
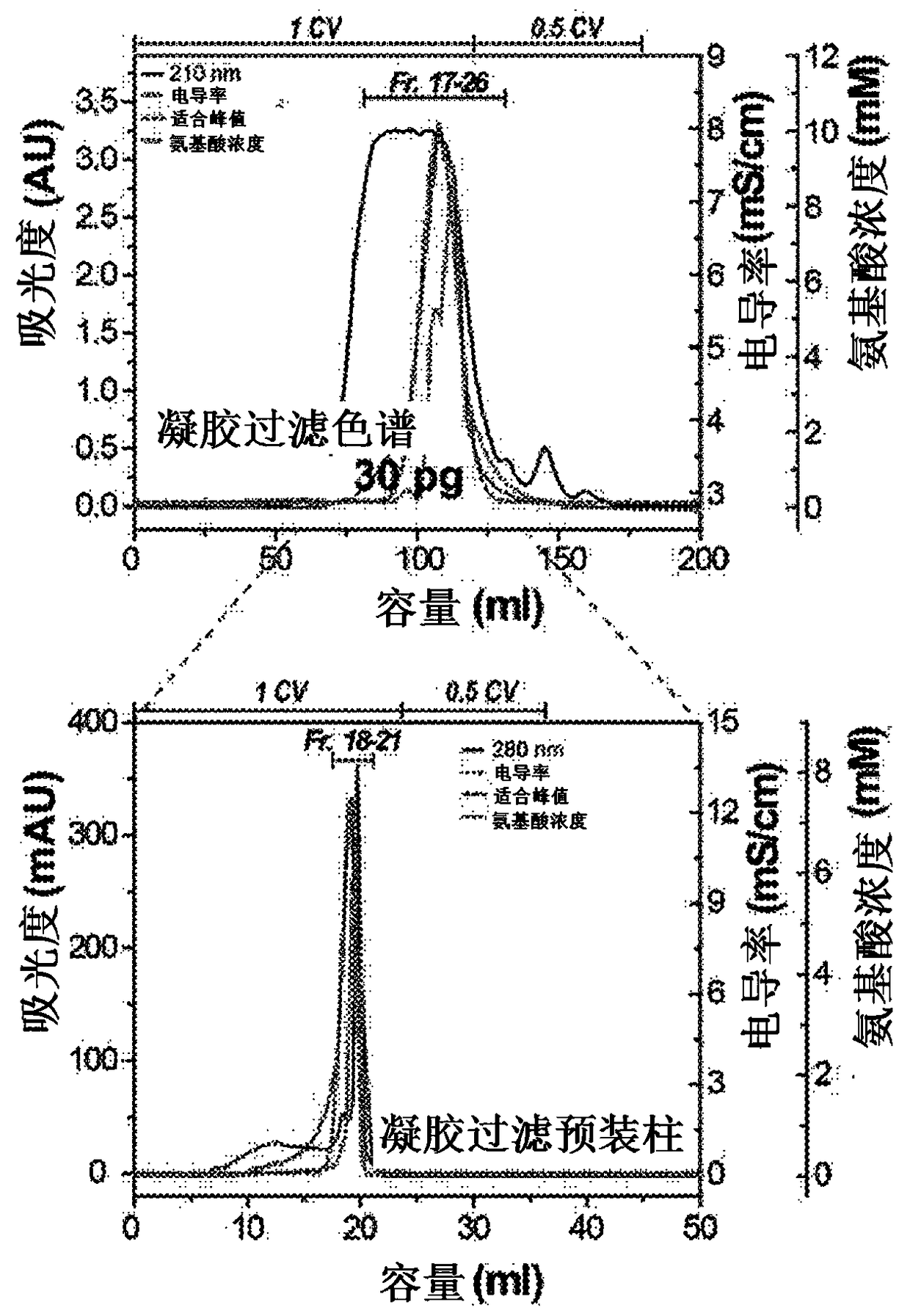
[0081] After that, the above-mentioned keratin hydrolyzate was subjected to the following operation: After primary filtration of the decomposed chicken feather residue with filter paper (5 μm, No. 20, Hyundai Micro, Korea), it was centrifuged at 10,000×g at 4°C 20 minutes, and the supernatant was recovered. The recovered supernatant is used as a sample in the separation and purification process of functional ultra-low molecular weight keratin peptides...
Embodiment 2
[0088] Example 2 . Cytotoxicity assay of keratin peptides in human skin fibroblasts and gene expression induced by ultraviolet B Inhibitory effect of metalloproteinase-1 expression
[0089] In order to examine the cytotoxicity of the keratin peptide prepared by the method of Example 1 above, MTT assay was performed using human dermal fibroblast.
[0090] First, human fibroblasts were dispensed into 96-well plates such that 5×10 3 cell / well, using Dulbecco's Modified Eagle's Medium (Dulbecco's Modified Eagle's Medium) mixed with 10% fetal bovine serum (FBS) and penicillin-streptomycin (GIBCO Invitrogen, Auckland, NZ) sMedium, DMEM), at 37°C, 5% CO 2 Under the condition of , cultured for 6 hours. 200 mg of 3-(4,5-dimethylthiazol-2)-2,5-diphenyltetrazolium bromide (MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5 -diphenyl-tetrazolium bromide) powder was dissolved in 40 ml of phosphate-buffered saline (PBS), and then filtered to prepare a 5 mg / mL MTT solution. Six hours after the ...
Embodiment 3
[0098] Embodiment 3. Amino Acid Sequence Identification and Synthesis of Keratin Peptides
[0099] As mentioned above, in order to clarify the amino acid sequence of the keratin peptide exhibiting this activity in the No. 2 and KP7 samples whose anti-aging ability and wrinkle improvement ability were confirmed, peptide identification by LC-MS / MS method was carried out .
[0100] Specifically, the chicken (Gallus gallus ver. 5.0) feather keratin sequence (feather keratin sequence) was obtained from the Genbank (Genbank) in order to be used for the database construction of keratin peptide mapping (mapping), and through AW- Transcript analysis of strain 1 confirmed the selection of proteases involved in chicken feather decomposition and peptide cleavage sites, and a database for LC-MS / MS results analysis was constructed on this basis. Electrospray quadrupole time-of-flight (ESI-Q-TOF, produced by Thermo (Dionex) of the United States, model: UHPLC Ultimate 3000, operating system...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com