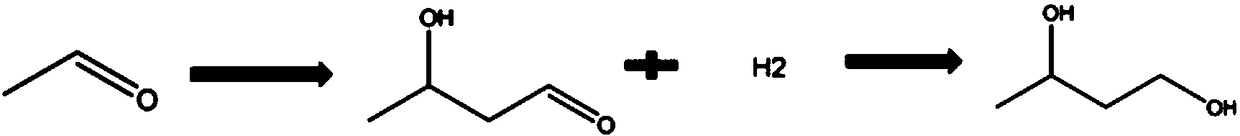
Preparation method of 1, 3-butylene glycol
A technology of butanediol and hydroxybutyraldehyde, applied in the chemical industry, can solve the problems of pungent odor, unsatisfactory yield and purity of 1,3-butanediol, and reduce side reactions, avoid deep condensation and dehydration The effect of high reaction and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Mix acetaldehyde and water evenly at a mass ratio of 7:1 and transport to the material pretreatment tank, keep the temperature at 15°C, and carry out under nitrogen protection;
[0035] (2) packing the loaded solid basic catalyst in a fixed bed, wherein the carrier is an ion exchange resin, the active component is tetrabutylammonium bromide, and the temperature is constant to 15° C.;
[0036] (3) the acetaldehyde solution in the step (1) is pumped into the fixed-bed reactor;
[0037] (4) In the fixed-bed reactor, the acetaldehyde aqueous solution undergoes an aldol condensation reaction under the catalysis of a supported solid basic catalyst. During this process, the reaction temperature is controlled at 15°C to 20°C, the pH value is kept at 13, and the reaction residence time is 4min. The crude product mainly contains unreacted acetaldehyde (15%-20%), 3-hydroxybutyraldehyde (70%-80%), a small amount of crotonaldehyde (0.5%-1%), other impurities and water;
[0038]...
Embodiment 2
[0045] (1) Mix the acetaldehyde and water evenly according to the mass ratio of 8:1, then transport to the material pretreatment tank, keep the temperature at 20°C, and carry out under the protection of nitrogen;
[0046] (2) Packing the loaded solid basic catalyst in a fixed bed, wherein the carrier is activated carbon, the active components are potassium and cerium (mass ratio 20:1), and the temperature is constant to 15° C.;
[0047] (3) the acetaldehyde solution in the step (1) is pumped into the fixed-bed reactor;
[0048] (4) In the fixed-bed reactor, the acetaldehyde aqueous solution undergoes an aldol condensation reaction under the catalysis of a supported solid basic catalyst. During this process, the reaction temperature is controlled at 20°C to 25°C, the pH value is kept at 12, and the reaction residence time is 5min. The crude product mainly contains unreacted acetaldehyde (15%-20%), 3-hydroxybutyraldehyde (70%-80%), a small amount of crotonaldehyde (0.5%-1%), oth...
Embodiment 3
[0056] (1) Mix acetaldehyde and water evenly at a mass ratio of 9:1 and transport to the material pretreatment tank, keep the temperature at 25°C, and carry out under nitrogen protection;
[0057] (2) packing the loaded solid basic catalyst in a fixed bed, wherein the carrier is aluminum oxide, the active components are potassium and cesium (mass ratio 20:1), and the temperature is constant to 25°C;
[0058] (3) the acetaldehyde solution in the step (1) is pumped into the fixed-bed reactor;
[0059] (4) In a fixed-bed reactor, an aldol condensation reaction occurs in an aqueous solution of acetaldehyde under the catalysis of a loaded solid basic catalyst. The process controls the reaction temperature at 25° C., maintains a pH value of 11, and has a residence time of 6 minutes to obtain a crude product. It mainly contains unreacted acetaldehyde (15%-20%), 3-hydroxybutyraldehyde (70%-80%), a small amount of crotonaldehyde (0.5%-1%), other impurities and water;
[0060] (5) The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com