Drug eluting balloon catheter and preparation method thereof
A balloon catheter and drug technology, applied in the direction of balloon catheters, catheters, etc., can solve the problems of coating shedding, limiting the use range of devices, and large outer diameters of devices, so as to improve safety, non-toxic safety, and prevent blood washout effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The second object of the present invention is to provide a method for preparing the above-mentioned drug-eluting balloon catheter, which includes:
[0028] S1. Obtain a coating solution for preparing a dense drug layer.
[0029]In this step, you can directly prepare the coating solution by yourself, or you can directly purchase the prepared coating solution, which is not limited here, but in order to ensure that it meets the above requirements for drug-eluting balloon catheters, the coating solution can be evenly attached On the surface of the balloon body, it is easy to form. In a preferred embodiment of the present invention, the mass concentration of the active drug in the coating solution is 1:500g / ml-1:50g / ml, such as 1:500g / ml, 1: 400g / ml, 1:350g / ml, 1:300g / ml, 1:250g / ml, 1:200g / ml, 1:150g / ml, 1:100g / ml or 1:60g / ml, etc. The mass ratio of the active drug to the carrier in the dense drug layer is 20:1-1:2, for example, the mass ratio of the active drug to the carr...
Embodiment 1
[0041] A drug-eluting balloon catheter made by the following method:
[0042] 100mg of paclitaxel, 40mg of iohexol, and 10ml of absolute ethanol were prepared as a coating solution, and the coating solution was inhaled into an airtight syringe matched with an ultrasonic atomizing sprayer.
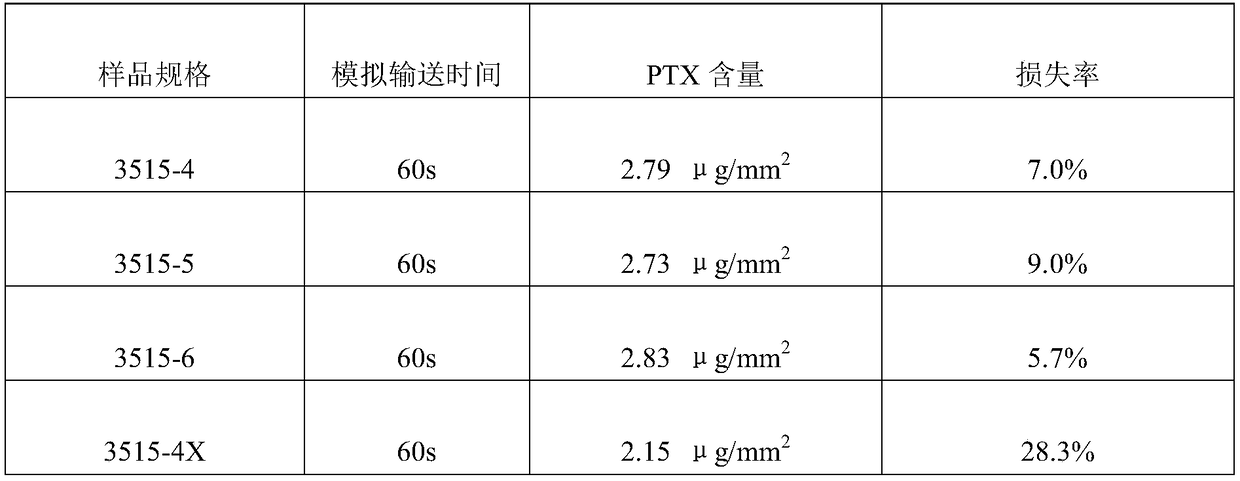
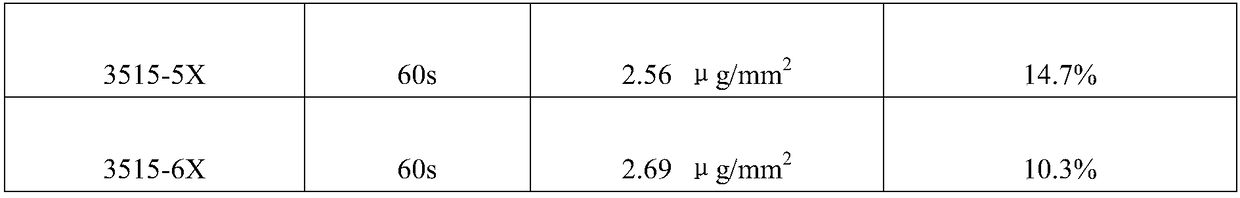
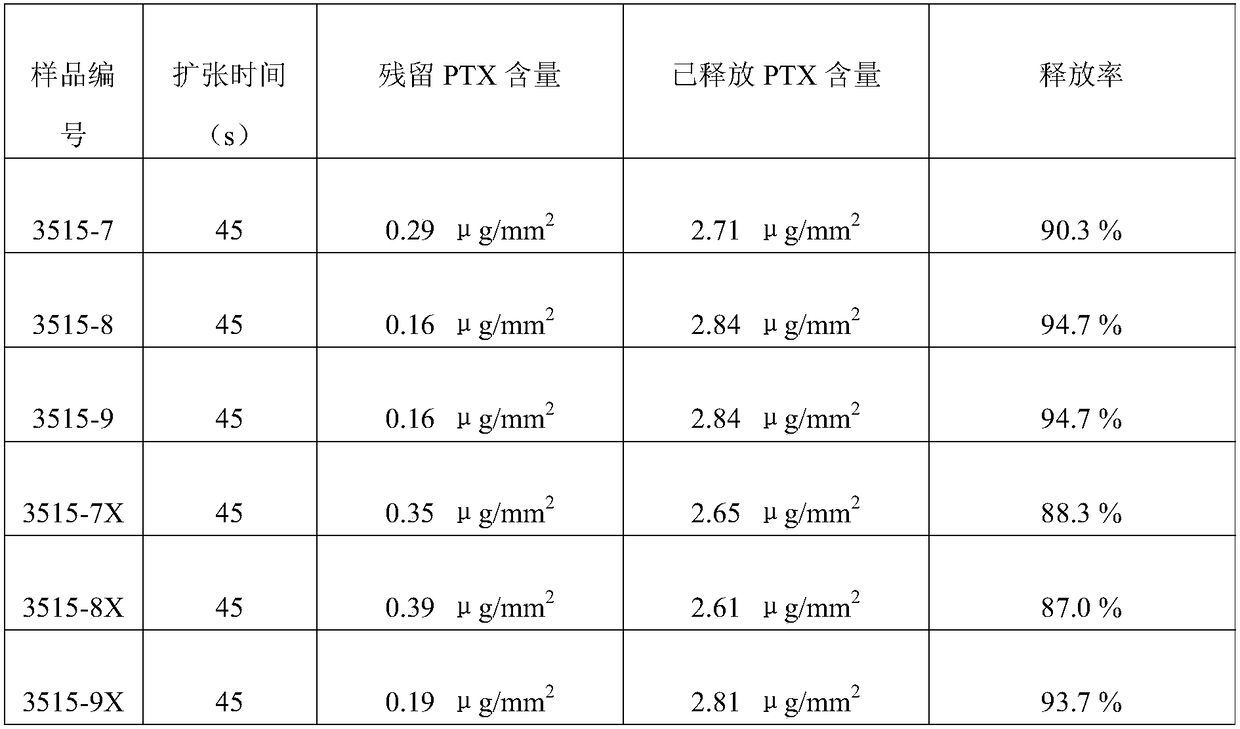
[0043] Process 3525 balloon body (diameter 3.5mm, effective length 25mm), the inner diameter is 0.70mm, the outer diameter is the balloon tube of 1.10mm (being the mixture of Pebax and Nylon12, wherein Pebax content is 80%, Nylon12 content is 20%, weight) into the balloon forming machine, and start the multi-stage balloon forming program. The forming machine will automatically use the drawing speed of 1.0-10mm / s and the heating temperature of 50°C-125°C in the 8 programs set in advance. The balloon tube starts the balloon body molding process. Here, the parameters of the balloon molding machine are different from those of common dilatable balloons. The molding temperature is 5°C higher tha...
Embodiment 2
[0047] A drug-eluting balloon catheter made by the following method:
[0048] 80mg of paclitaxel, 40mg of iopamidol and 13ml of tetrahydrofuran were prepared into a coating solution, and the coating solution was inhaled into the airtight syringe matched with the ultrasonic atomizing sprayer.
[0049] The 3512 balloon body (diameter 3.5mm, effective length 12mm) was obtained according to the method of Example 1, the front end of the 3512 balloon body was folded into 3 wings, and the folded balloon body was filled with clean compressed air with a pressure of 2.5Atm and used a micro The hemostatic clip seals the proximal inflation port.
[0050] Use an ultrasonic atomization spraying machine to spray the front end of the balloon body. After each spraying, perform an air-drying process on the front end of the balloon body. After the previous layer is air-dried, perform the next spraying, and repeat the spraying several times until the balloon The concentration of active drug on t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com