2,3-dihydro-1h-quinolin-4-one thiosemicarbazone derivatives and its preparation method and application
A technology of thiosemicarbazone and derivatives, which is applied in the field of medicine and achieves the effects of mild conditions, high efficiency and inhibition of tumor cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
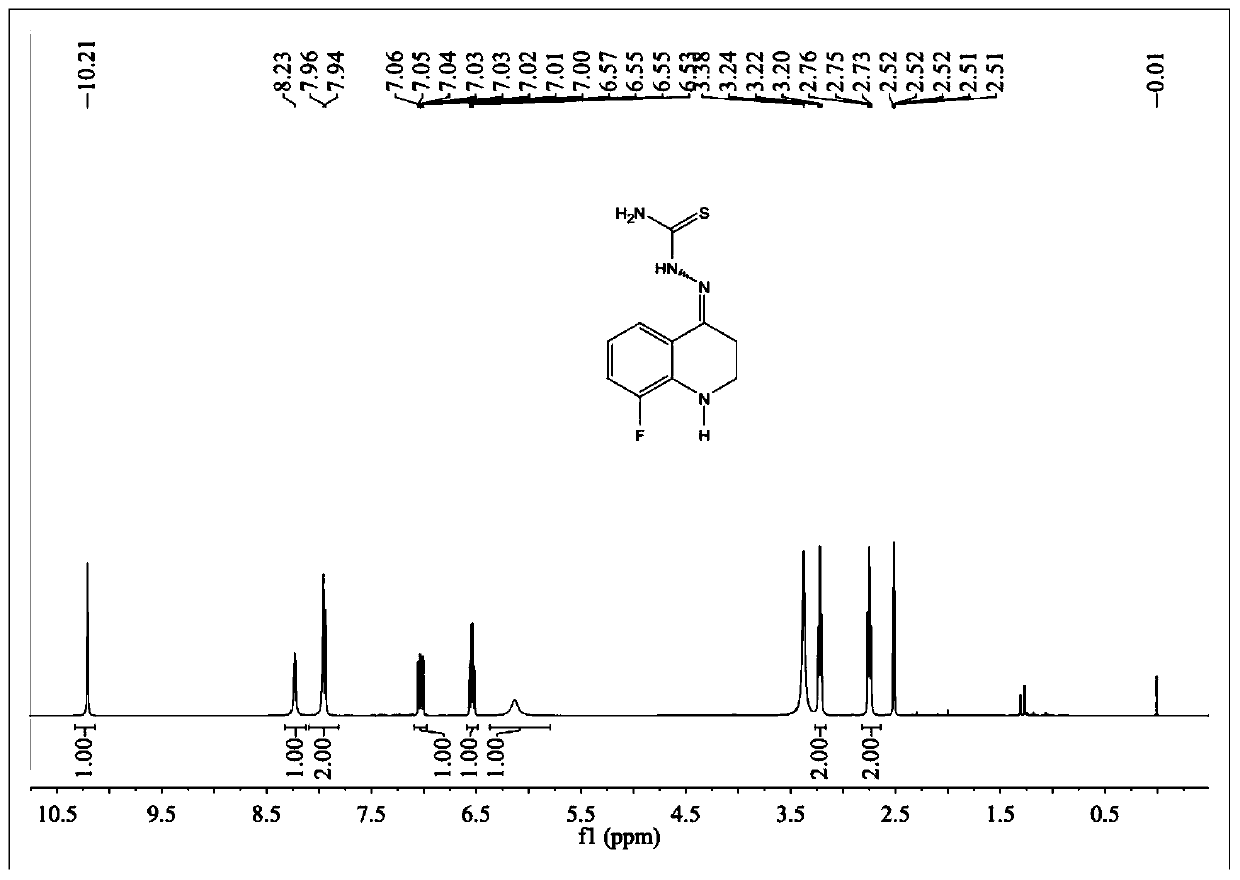
[0076] Example 1 Synthesis of 8-fluoro-2,3-dihydro-1H-quinolin-4-one thiosemicarbazone
[0077] (1) Synthesis of 3-(o-fluoroanilino)propionic acid
[0078] Add 13.74mmol of 2-fluoroaniline, 16.48mmol of methyl acrylate, and 3.435mmol of glacial acetic acid to a 250mL round-bottomed flask, and heat to reflux at 80°C for 8h; then add 58mmol of sodium hydroxide, 25mL of water, and 25mL of methanol in sequence, Under the condition of 94°C, the hydrolysis reaction occurred in the round bottom flask for 20 hours; the reaction was tracked and monitored by TLC, after the reaction was completed, cooled to room temperature (20-25°C), adjusted the pH value to 4-5 with concentrated hydrochloric acid, and then used 25mL acetic acid Ethyl is extracted until the organic layer is colorless; then, the rotary evaporator removes the solvent to obtain a white solid product, which is 3-(o-fluoroanilino)propionic acid, and its productive rate is 64%. Its structural formula is:
[0079] NMR 1 H-...
Embodiment 2
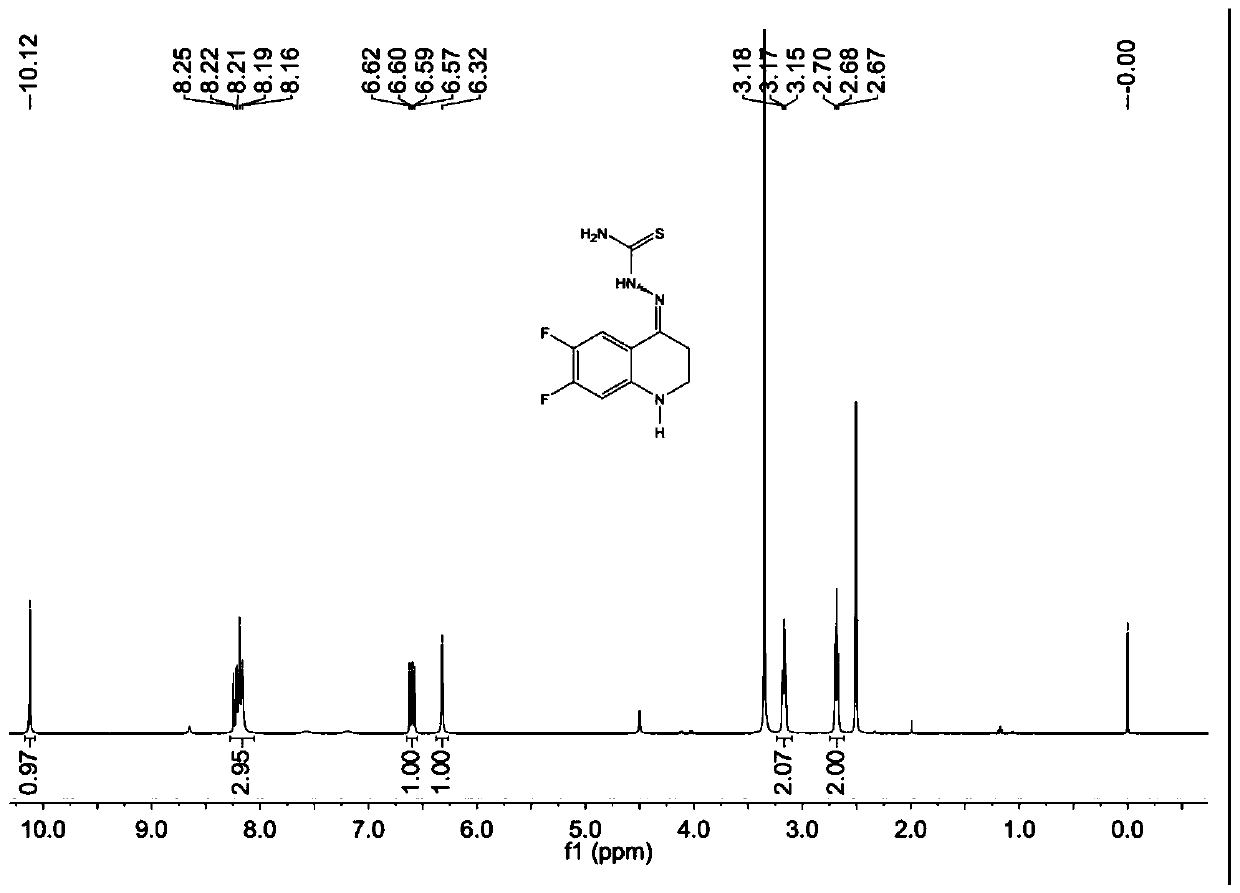
[0088] Example 2 Synthesis of 6,7-difluoro-2,3-dihydro-1H-quinolin-4-one thiosemicarbazone
[0089] (1) Synthesis of 3-(3,4-difluoroanilino)propionic acid
[0090] According to the preparation method of step (1) in Example 1, other conditions remain unchanged, and an equivalent amount of 3,4-difluoroaniline is used instead of 2-fluoroaniline to obtain a brown oily liquid product, which is 3-(3,4- Difluoroanilino) propionic acid, its productive rate is 40%, and its structural formula is:
[0091] NMR 1 H-NMR (CDCl 3 ,400MHz): δ6.95(1H,ArH,m), 6.43(2H,ArH,m), 6.29(1H,ArH,d), 3.41(2H,CH 2 ,t), 2.65(2H,CH 2 ,t).
[0092] (2) Synthesis of 6,7-difluoro-2,3-dihydro-1H-quinolin-4-one
[0093]According to the preparation method of step (2) in Example 1, with other conditions unchanged, an equivalent amount of 3-(3,4-difluorophenylamino)propionic acid was used instead of 3-(o-fluorophenylamino)propionic acid to obtain the product It is a yellow powder product, namely 6,7-difluo...
Embodiment 3
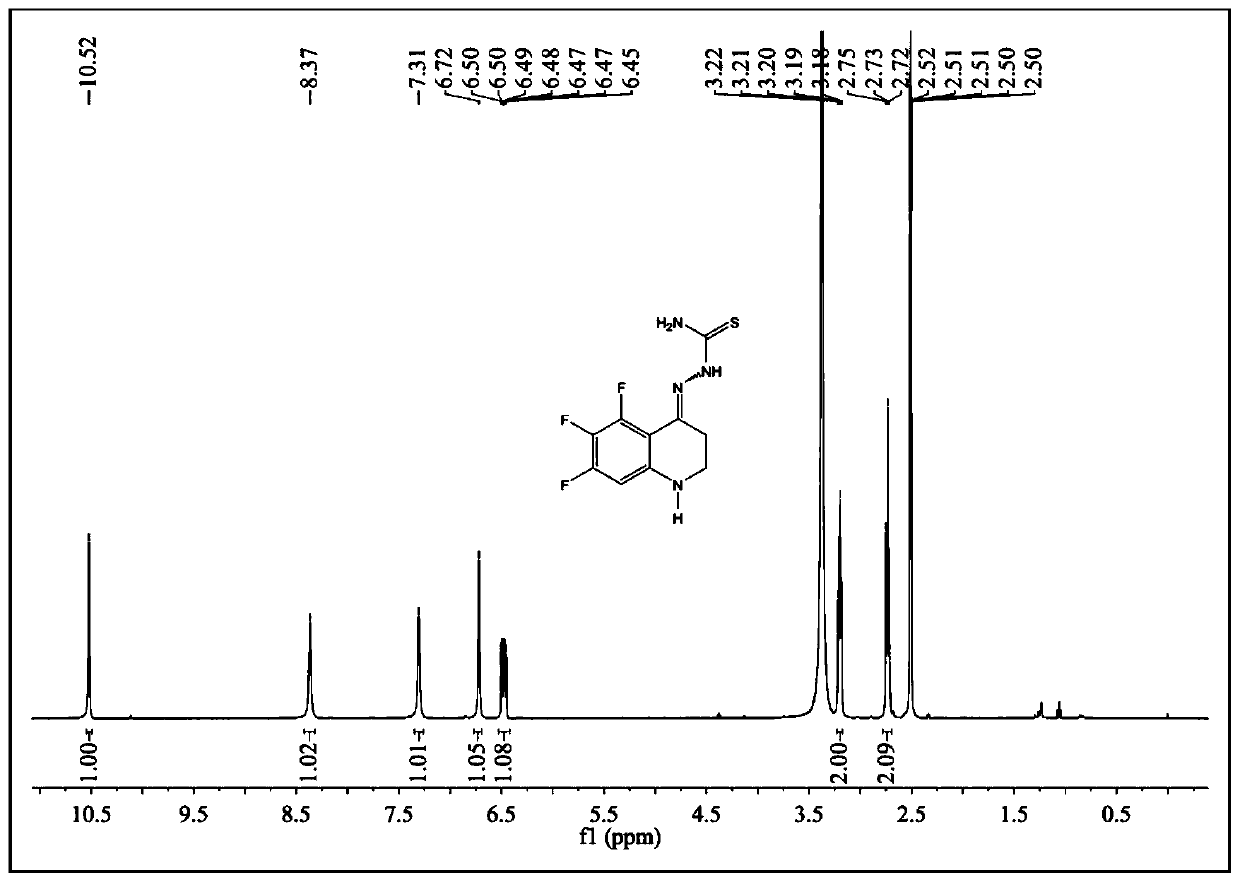
[0100] Example 3 Synthesis of 5,6,7-trifluoro-2,3-dihydro-1H-quinolin-4-one thiosemicarbazone
[0101] (1) Synthesis of 3-(3,4,5-trifluoroanilino)propionic acid
[0102] According to the preparation method of step (1) in Example 1, other conditions remain unchanged, and an equivalent amount of 3,4,5-trifluoroaniline is used instead of 2-fluoroaniline to obtain a reddish-brown oily product, which is 3-(3, 4,5-trifluoroanilino) propionic acid, its productive rate is 72.7%, and its structural formula is:
[0103] NMR 1 H-NMR (CDCl 3 ,400MHz): δ6.19(2H,ArH,dd), 3.39(2H,CH 2 , dd), 2.66 (2H, CH 2 ,t).
[0104] (2) Synthesis of 5,6,7-trifluoro-2,3-dihydro-1H-quinolin-4-one
[0105] According to the preparation method of step (2) in Example 1, other conditions remain unchanged, replace 3-(o-fluoroanilino) propionic acid with an equivalent amount of 3-(3,4,5-trifluoroanilino) propanoic acid, A brown powder product was obtained, which was 5,6,7-trifluoro-2,3-dihydro-quinolin-4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com