Folate receptor medium, optical excitation photosensitive drug conjugate and preparation method of optical excitation photosensitive drug conjugate
A technology of photosensitive drugs and folic acid receptors, which is applied in drug combinations, pharmaceutical formulations, photodynamic therapy, etc., can solve the problems that have not been reported in the literature of targeted photosensitive drug conjugates, achieve enhanced effects, prolong circulation time, Avoid the effect of devouring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
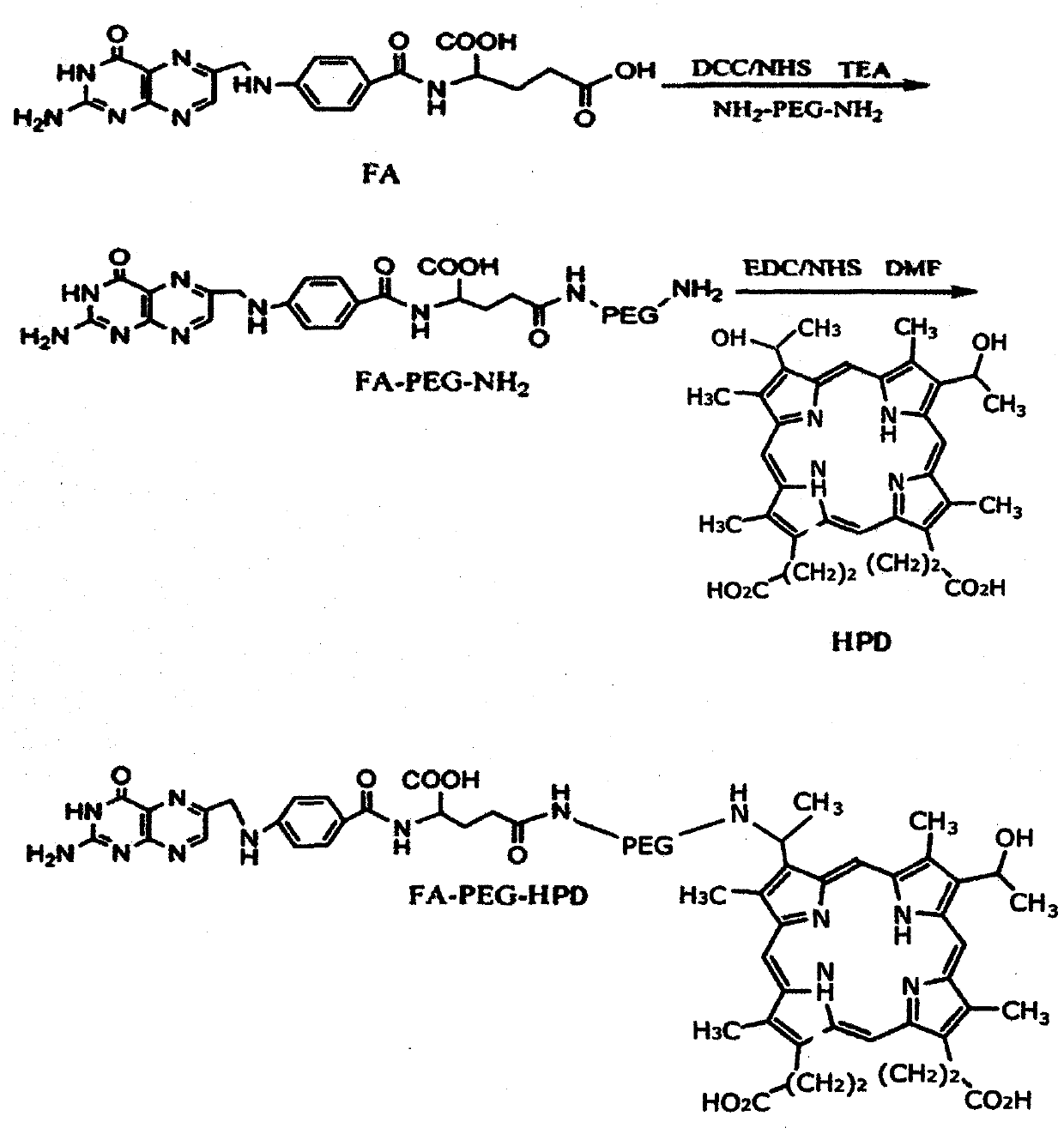
[0021] Example 1 Preparation of folic acid-polyethylene glycol-hematoporphyrin conjugate, and synthesis of folic acid-polyethylene glycol-photosensitive coupling agent.
[0022] 1) Synthesis of polyethylene glycol for the function of folic acid.
[0023] Weigh the double-end amino polyethylene glycol (NH 2 -PEG-NH 2 , M=3200Da) 640mg was dissolved in 10ml anhydrous DMSO, 2ml DMSO solution dissolved with folic acid FA and NHS was added, then DCC, TEA (molar dosage of each substance NH) were added in turn 2 -PEG-NH 2 : FA: NHS: DCC: TEA = 1: 1: 1.5: 1.5: 5), under nitrogen protection, stirred at room temperature, and reacted in the dark for 12 h, the reaction mixture was diluted with 20 ml of deionized water, and centrifuged at 500 rpm to remove the secondary The product dicyclohexylurea was added with 10 ml of acetone to remove unreacted folic acid. The supernatant was put into a dialysis tube with a molecular weight cut-off of 3000 Da, diluted with deionized water for 48 ho...
Embodiment 2
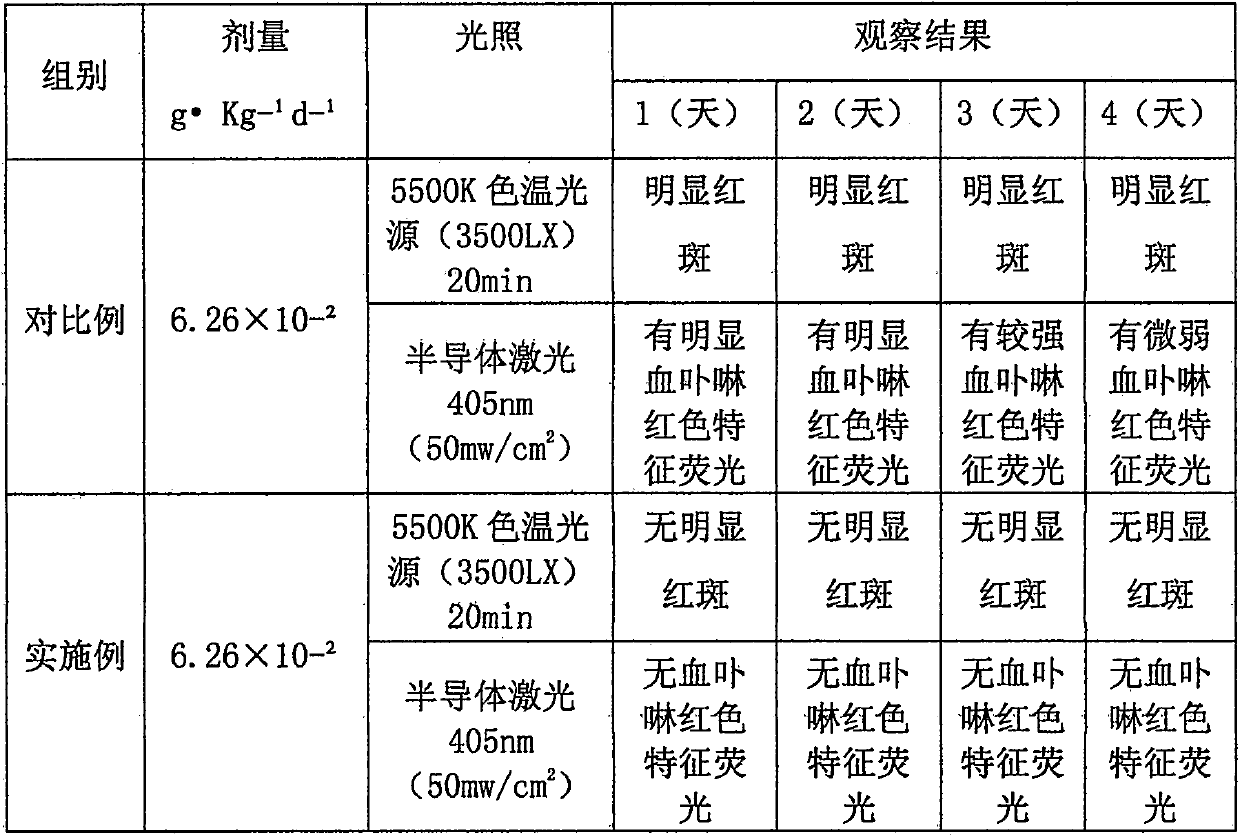
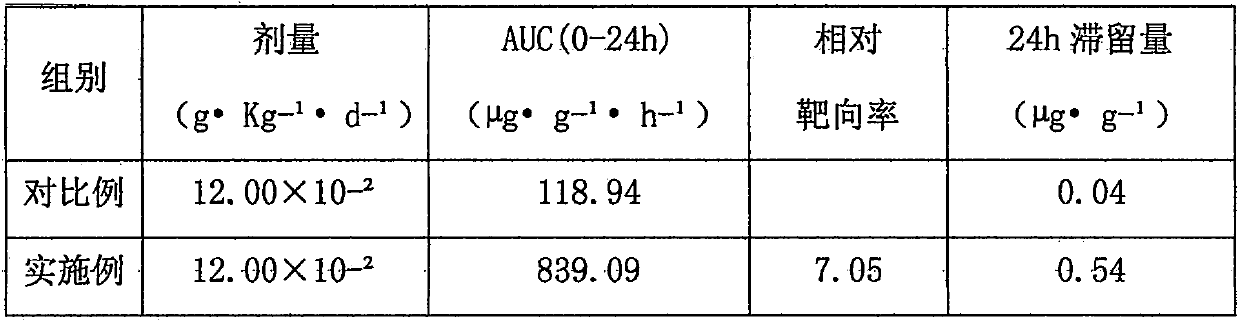
[0026] Example 2 Observation of phototoxic side effects and targeting effect in tumor tissue.
[0027] 1) Phototoxicity side reaction test
[0028] Take hematoporphyrin injection (Chongqing Huading Now Biopharmaceutical Co., Ltd.) as a comparative example. Twenty ICR mice, half male and half male, were randomly divided into 2 groups with 10 mice in each group. The mice in each group were given the medicines of the examples and comparative examples through the tail vein (the dosage calculated according to hematoporphyrin was 6.26 × 10). -2 g·Kg -1 ·d -1 ). Before administration, the backs of the mice were dehaired, and the dehaired area was 2cm×2cm. 1h after administration, the two groups of mice were placed under the light condition of 5500K color temperature light source (approximately outdoor afternoon sunlight) 3500LX for 20min, and the back skin of the mice was used as the main observation area, and the comparative examples and pairs were observed at different times. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com