Pitavastatin calcium intermediate preparation method
A technology for pitavastatin calcium and intermediates, which is applied in the field of chemical substance preparation, can solve problems such as difficulty in obtaining pure pitavastatin calcium, bottlenecks in industrial production, expensive atom economy, etc., and achieve good stereoselectivity, novel route, The effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
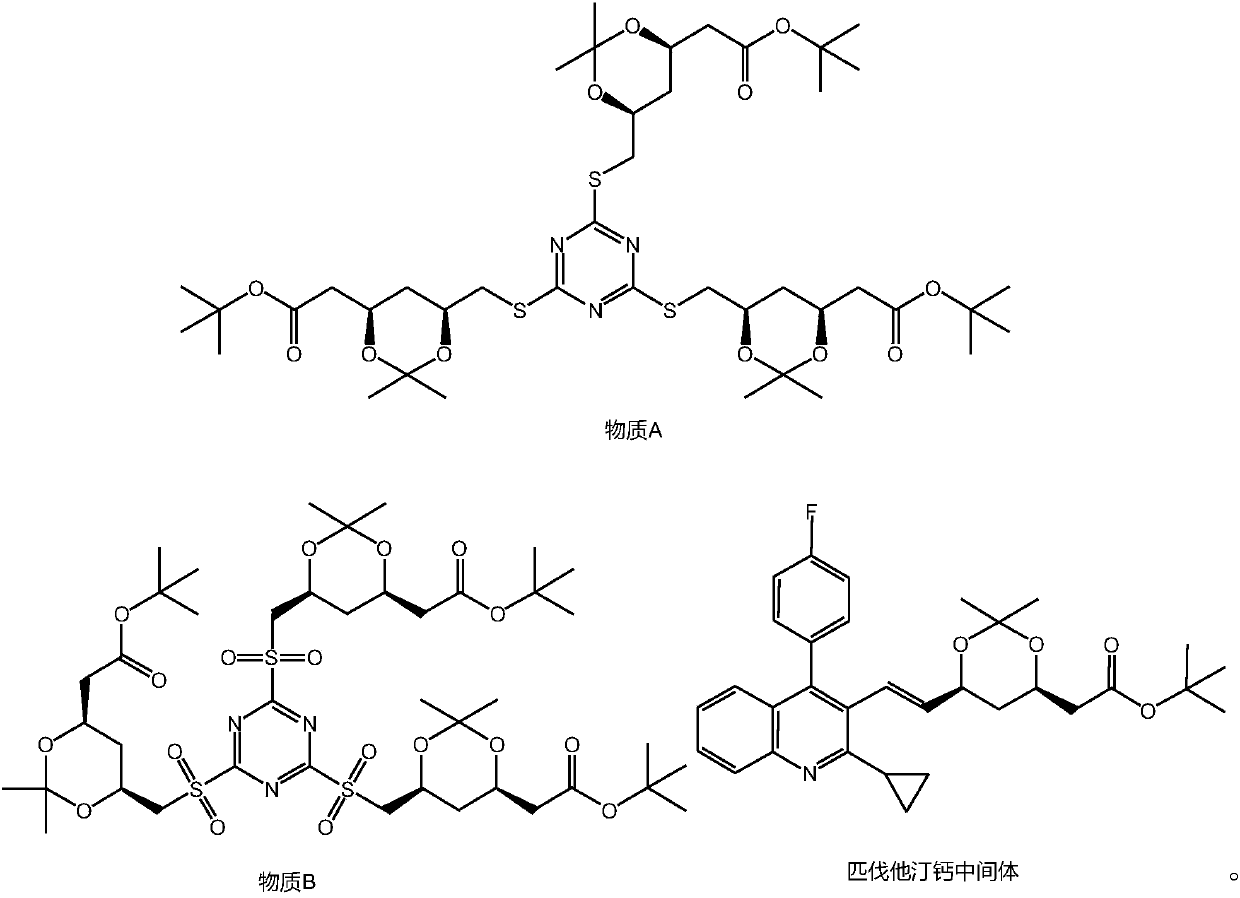
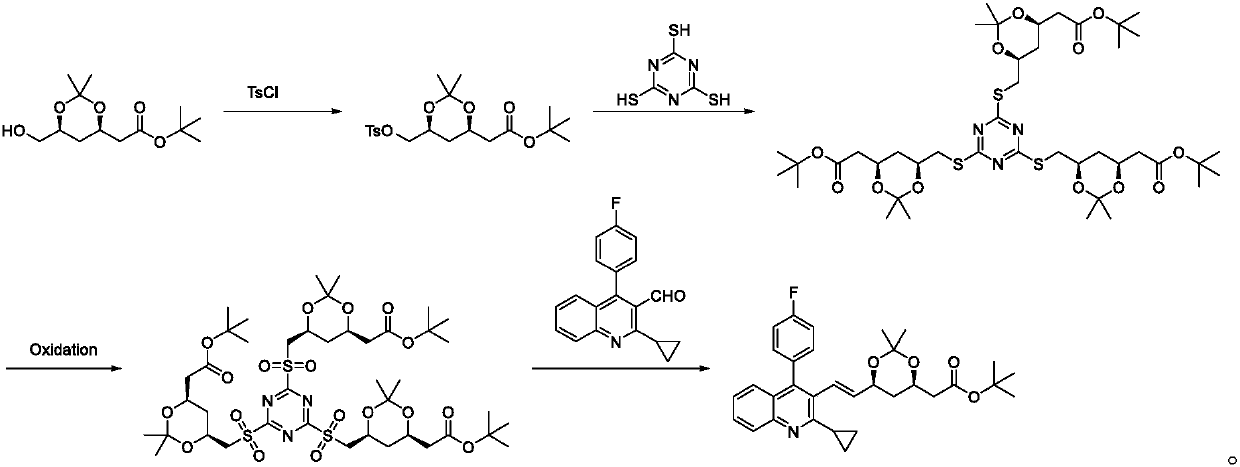
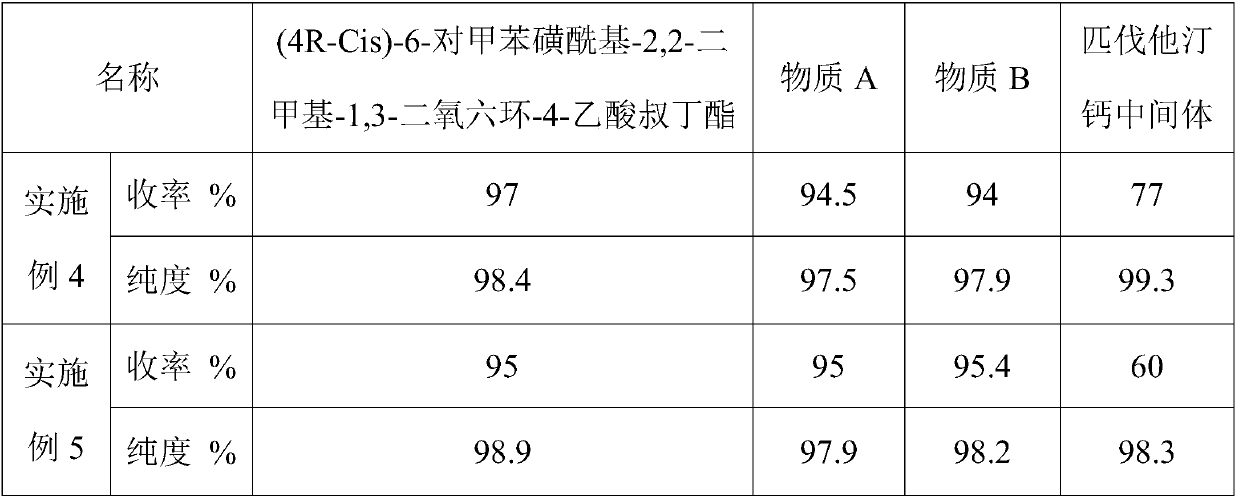
[0059] A preparation method of pitavastatin calcium intermediate, comprising the steps of: (4R-Cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl Ester and p-toluenesulfonyl chloride are sulfonylated under the action of a catalyst to obtain (4R-Cis)-6-p-toluenesulfonyl-2,2-dimethyl-1,3-dioxane-4-acetic acid tertiary Butyl ester; then react with trimercapto-s-triazine under the action of basic catalyst to obtain substance A; then oxidize to obtain substance B through the action of oxidizing agent; finally with 2-cyclopropyl-4-(4-fluorophenyl) quinoline- 3-Formaldehyde reacted under the catalysis of sodium hydride to obtain pitavastatin calcium intermediate.
Embodiment 2
[0061] A preparation method of pitavastatin calcium intermediate, comprising the steps of:
[0062] Mix (4R-Cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester, dimethylamine, dichloromethane and cool down to - 5°C, dropwise add the dichloromethane solution of p-toluenesulfonyl chloride, during the dropwise addition, keep the temperature not exceeding 5°C, reflux for 24 hours after the dropwise addition, wash with water, extract the organic phase with dichloromethane, dry, and concentrate to obtain (4R -Cis)-6-p-toluenesulfonyl-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate, wherein (4R-Cis)-6-hydroxymethyl-2 , The molar ratio of 2-dimethyl-1,3-dioxane-4-tert-butyl acetate to dimethylamine is 1:1.5, (4R-Cis)-6-hydroxymethyl-2,2- The molar ratio of dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester to p-toluenesulfonyl chloride is 1:1.5;
[0063] In a nitrogen atmosphere, trimercapto-s-triazine, sodium hydroxide, 1,4-dioxane, (4R-Cis)-6-p-toluenesulfo...
Embodiment 3
[0067] A preparation method of pitavastatin calcium intermediate, comprising the steps of:
[0068] Mix (4R-Cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester, pyridine and dichloromethane, cool to -2°C , dropwise add a dichloromethane solution of p-toluenesulfonyl chloride, keep the temperature not exceeding 5°C during the dropwise addition, reflux for 18h after the dropwise addition, wash with water, extract the organic phase with dichloromethane, dry, and concentrate to obtain (4R-Cis )-6-p-toluenesulfonyl-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester, wherein, (4R-Cis)-6-hydroxymethyl-2,2 -Dimethyl-1,3-dioxane-4-tert-butyl acetate to pyridine in a molar ratio of 1:2, (4R-Cis)-6-hydroxymethyl-2,2-dimethyl- The molar ratio of 1,3-dioxane-4-acetic acid tert-butyl ester to p-toluenesulfonyl chloride is 1:1.3;
[0069] In a nitrogen atmosphere, trimercapto-s-triazine, potassium hydroxide, 1,4-dioxane, (4R-Cis)-6-p-toluenesulfonyl-2,2-dimeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com