Response type small molecular peptide nano drug-loading carrier
A nano-drug-carrying and carrier technology, which can be applied to inactive components of polymer compounds, peptides, microcapsules, etc., can solve problems such as refractory degradation and immune response, achieve low synthesis cost, convenient purification, and avoid potential biological toxicity and immunity. source effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Ac-VVVVVVKKK-NH 2 Preparation of peptides
[0047] Step 1. Distill N,N-dimethylformamide (DMF) and piperidine (Piperidine) solvents
[0048] The purchased DMF solution was distilled under reduced pressure at 60°C to obtain pure DMF solvent; the purchased piperidine was heated to reflux for 1-2 hours, and the boiling point temperature (106°C) fraction was received to obtain pure piperidine solvent.
[0049] Step 2, preparation of amino acid, resin, activator, capping agent, deprotecting agent
[0050] Calculate and prepare 0.25mM Ac-VVVVVVKKK-NH on the polypeptide solid-phase synthesizer 2 Amounts of amino acids and other reagents required:
[0051] Lys (lysine): 1.04g dissolved in 11mL DMF;
[0052] Val (valine): 2.18g dissolved in 32mL DMF;
[0053] Resin (loaded at 0.6mmol / g): 0.417g;
[0054] Activator: Diisopropylcarbodiimide (DIC): 17mL;
[0055] Activated base: 17-(Acetyloxy)-3-Methoxy-20-oxo-pregna-3,5-diene-6-carboxaldehyde (Oxyma, CAS No.: 57361...
Embodiment 2
[0061] Example 2: Ac-VVVVVVKKK-NH 2 Preparation of drug-loaded carrier
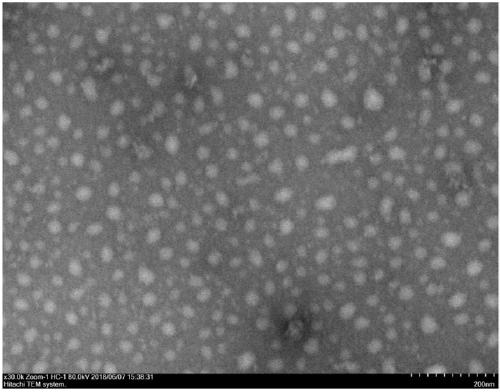
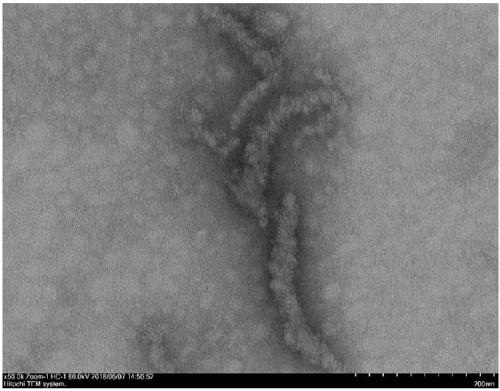
[0062] Weigh 0.519mg (0.5mmol / L) peptide Ac-VVVVVVKKK-NH 2 , adding 1 mL of PBS buffer solution, ultrasonication for 5 min, and standing at room temperature for 4 h, the observation results of TEM and SEM showed that nanoparticle self-assembly had been formed at this time ( figure 1 , figure 2 ).
Embodiment 3
[0063] Example 3: Ac-VVVVVVKKK-NH 2 Detection of self-assembled morphology of drug-loaded carriers in PBS buffer
[0064] The specific detection method is as follows:
[0065] Examination of self-assembled morphologies (TEM, SEM) in PBS buffer.
[0066] TEM: The model of TEM used in the experiment is JEM 1400Plus transmission electron microscope. Take 30 μL of the sample to be tested obtained in Example 2 and drop it on the PARAFILM sealing film to form a droplet, put the copper mesh covered with the carbon support film on the droplet to absorb for 30s to 3min, remove the copper mesh, and absorb the excess liquid on the surface with filter paper , and then use 2% uranyl acetate aqueous solution to negatively stain the carbon support membrane with the sample adsorbed for 1 to 3 minutes, absorb the excess dye solution with filter paper, leave it to dry at room temperature, and use TEM to test, the electron acceleration voltage It is 120KV. The results showed that the peptide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com