Autonomously illuminated Acinetobacter baumannii and construction method and application thereof
An Acinetobacter baumannii, self-luminous technology, applied in the field of genetic engineering, can solve problems such as not yet seen in Acinetobacter baumannii, and achieve the effects of strong stability, simple and fast operation, and high luminous intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
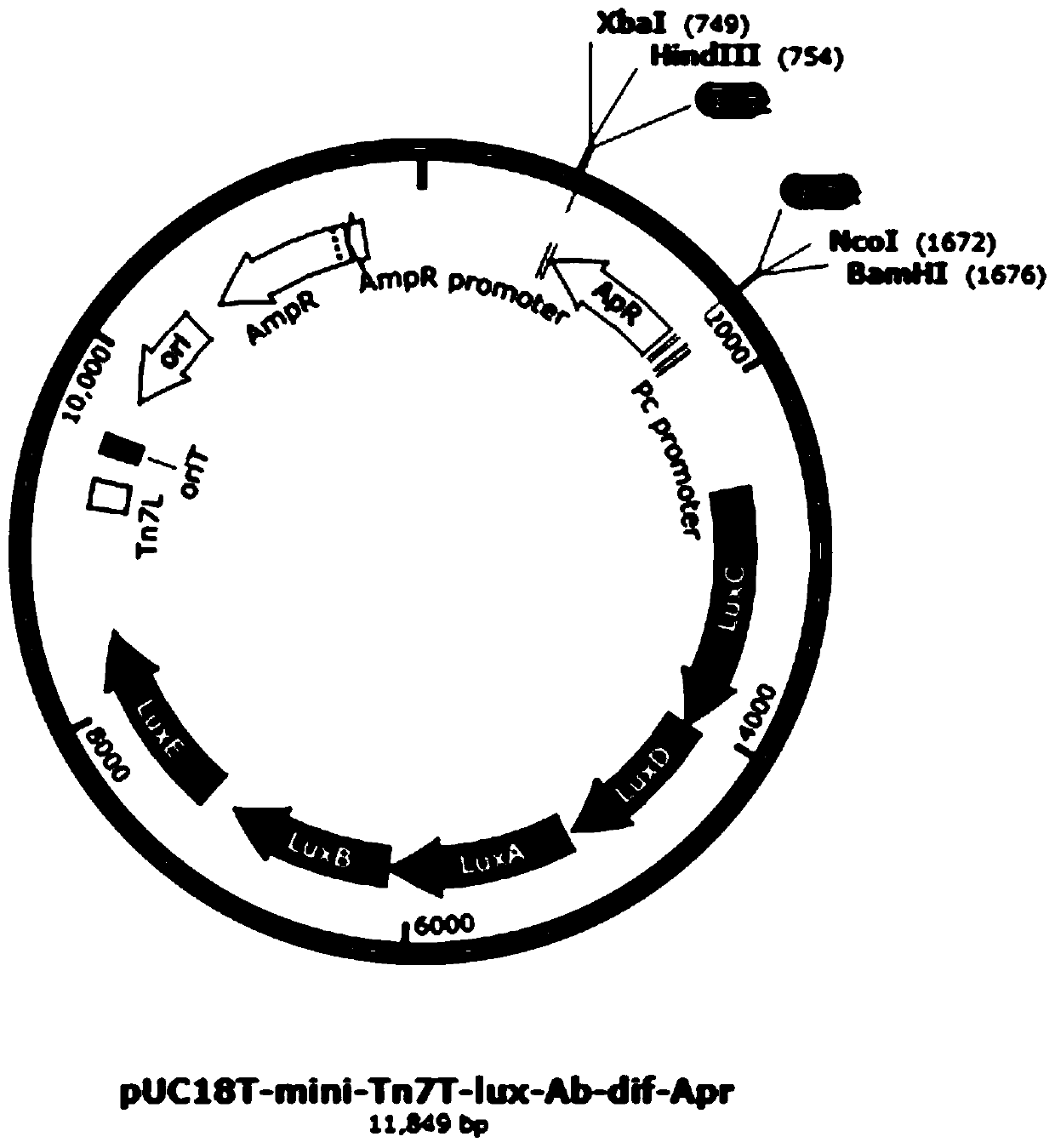
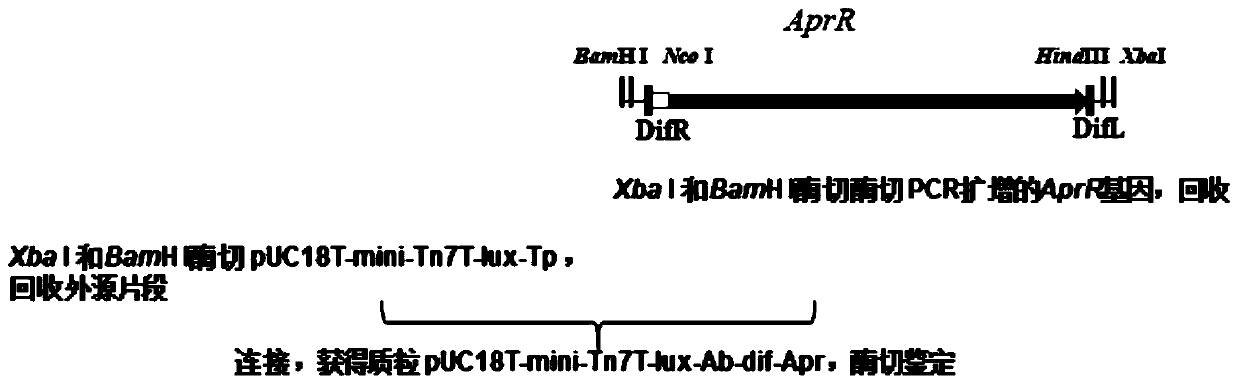
[0046] Example 1 Construction of transfer plasmid pUC18T-mini-Tn7T-lux-Ab-dif-Apr
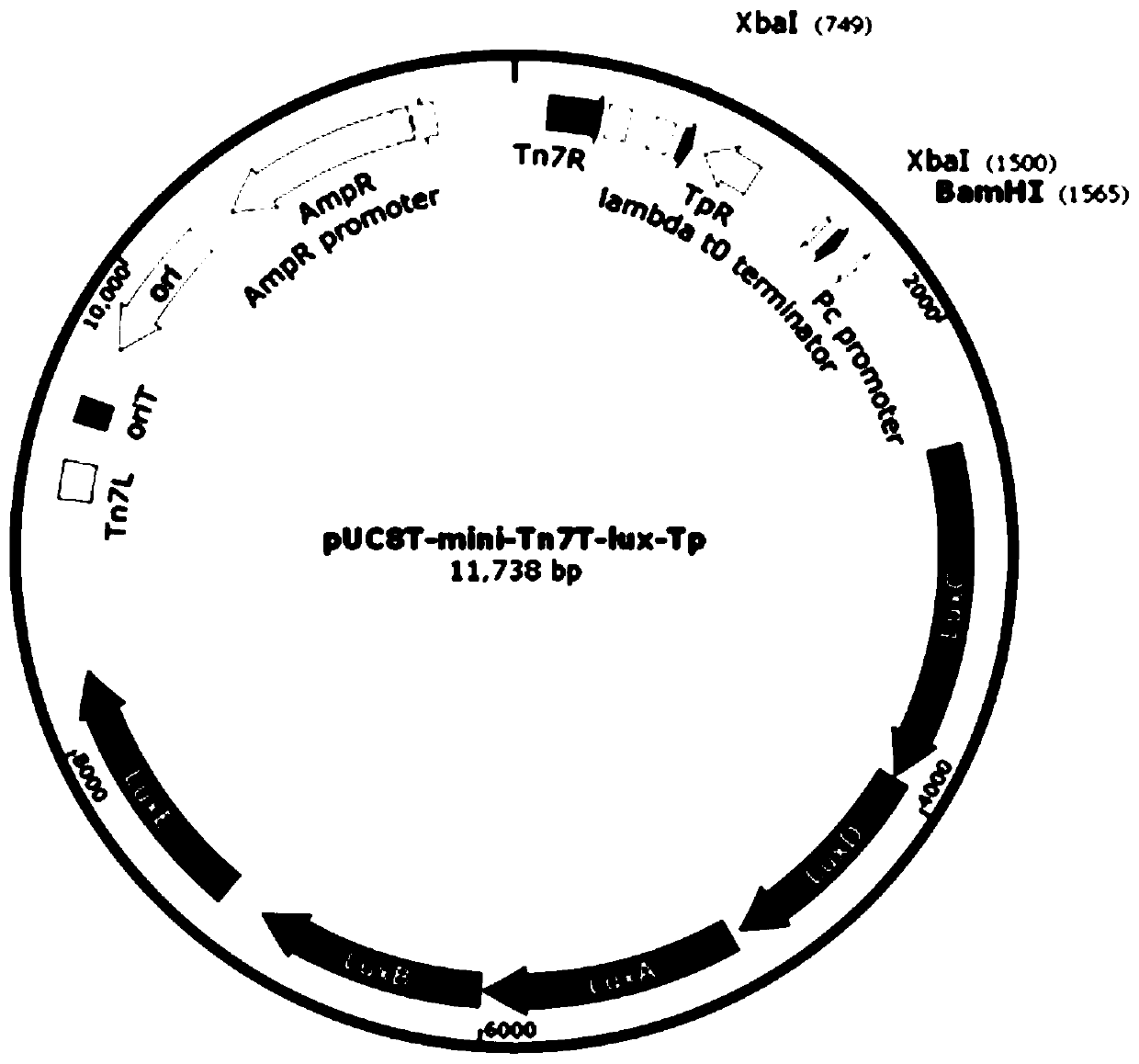
[0047] (1) According to the process image 3 The transfer plasmid pUC18T-mini-Tn7T-lux-Ab-dif-Apr was constructed. Such as figure 2 As shown, the plasmid pUC18T-mini-Tn7T-lux-Ab-dif-Apr contains the origin of replication (ori), ampicillin resistance gene (AmpR), and apramycin resistance gene (Apr) in a clockwise direction , the luciferase gene (LuxCDABE), the direct repeat sequences DifR and DifL located at both ends of the AprR gene. The functions of each component are as follows:
[0048] LuxCDABE: the luciferase gene, the enzyme gene required for luminescence, the expression of this gene allows the host bacteria to emit light autonomously.
[0049] Apramycin resistance gene (AprR): Apramycin resistance gene (Apr) is a selectable marker for screening to obtain the target strain; Apramycin resistance gene (Apr) in Acinetobacter baumannii After being expressed in Escherichia coli, the hos...
Embodiment 2
[0060] Example 2 A method for constructing an autonomous luminescent Acinetobacter baumannii without a resistance screening marker
[0061] 1. Electroconversion
[0062] (1) To prepare Acinetobacter baumannii competent cells, inoculate a single colony (Acinetobacter baumannii) to be transformed into 15mL LB medium, culture at 37°C with shaking at 200rpm until OD 600 At 0.4-0.8, room temperature, 12000g, centrifuge for 5min, collect the bacteria. Resuspend and wash the cells with 10 mL of 10% glycerol, centrifuge at 12000 g for 5 min at room temperature, pour off the residual liquid, and repeat twice. Resuspend bacteria in 500 μL of 10% glycerol and mix well. Aliquot the competent cells into 100uL / tubes, put them in a -80°C refrigerator, and save them for later use (it is best to prepare new competent cells each time).
[0063] (2) Transfer 100 μL of Acinetobacter baumannii competent cells to a 2 mm electroporation cuvette, add 50 ng of the transfer plasmid pUC18T-mini-Tn7T-...
Embodiment 3
[0085] Embodiment 3 verifies the luminescence stability of no resistance AlAb
[0086] 1) Subculture the non-resistant AlAb prepared in Example 2
[0087] a. Inoculate 5 mL of AlAb into LB liquid medium at a ratio of 1:10000, and culture it in a constant temperature shaker at 37°C at 200 rpm;
[0088] b. When the bacteria solution OD 600 When it reaches 0.7, inoculate 5 mL of the bacterial solution into LB liquid medium at a ratio of 1:10000, and place it in a constant temperature shaker at 37°C and 200 rpm for cultivation;
[0089] c. After repeating step b several times, spread the bacterial solution on a non-resistant LB plate, and place it in a constant temperature incubator at 37°C for cultivation, and observe after 24 hours.
[0090] 2) Statistical proportion of AlAb
[0091] When colonies grew on the plate, 200 single colonies were randomly picked and RLUs were detected with a luminescence detector. If the RLUs of the detected single colony are more than 5 times the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com