Perovskite oxide catalyst and preparation method and application thereof
A perovskite oxide and catalyst technology, applied in the field of electrochemical catalysis, can solve the problems of high price, poor stability, and low precious metal reserves, and achieve excellent oxygen evolution activity and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
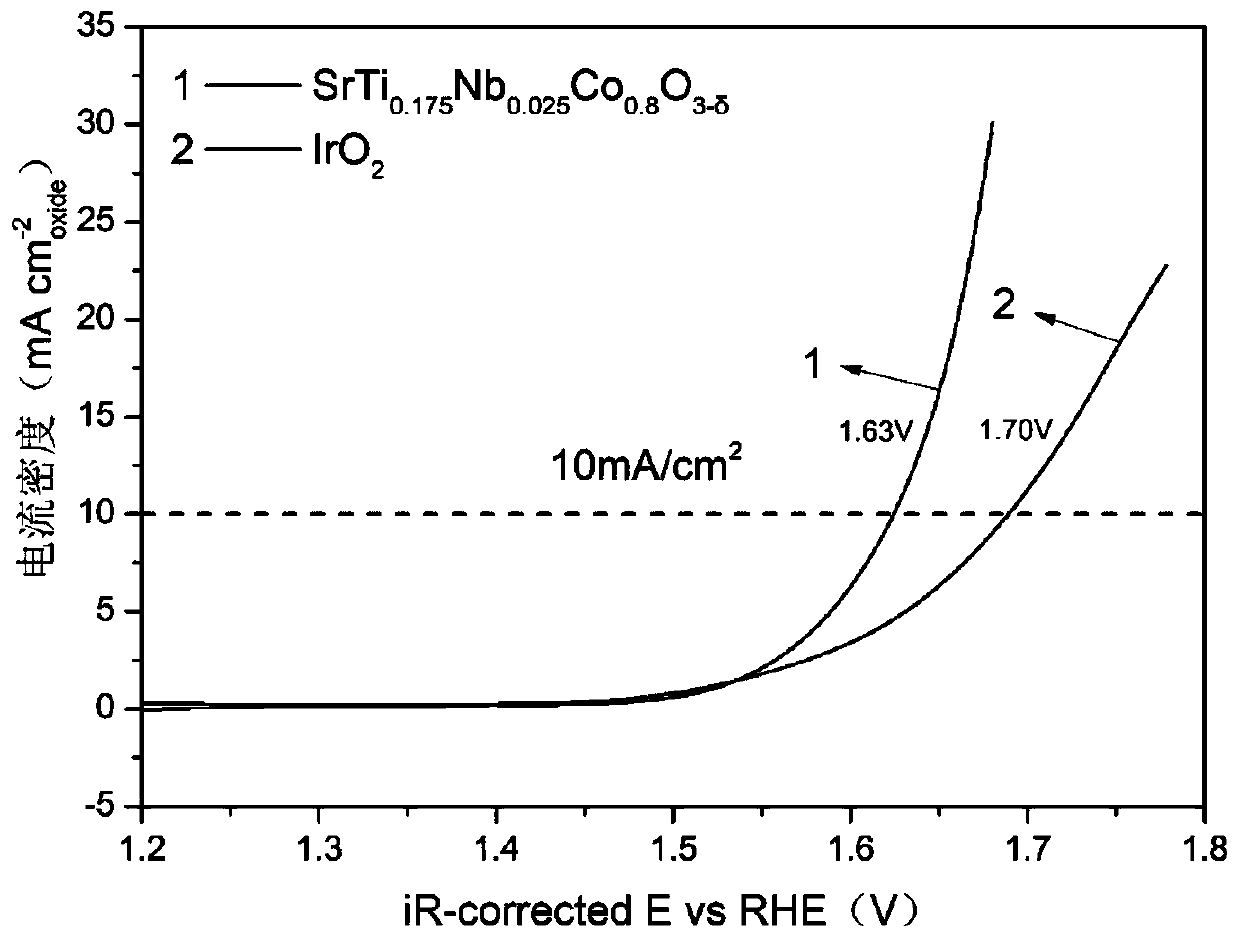
[0029] SrTi 0.175 Nb 0.025 co 0.8 o 3-δ Catalyst preparation and evaluation of oxygen evolution activity
[0030] SrTi 0.175 Nb 0.025 co 0.8 o 3-δ The catalyst powder is prepared by a sol-gel method. Take tetrabutyl titanate, niobium oxalate, strontium nitrate, and cobalt nitrate in stoichiometric ratio respectively, add citric acid (CA) and ethylenediaminetetraacetic acid (EDTA), add deionized water and heat on a magnetic stirrer to obtain For the corresponding solution, add ammonia water to adjust the pH value to about 7, continue to stir and heat until the solution becomes gelatinous, put the beaker in an oven at 250°C, and heat for 5 hours to obtain a solid precursor, and place the precursor in a muffle furnace, after calcination at 950°C for 5 hours, the desired SrTi 0.175 Nb 0.025 co 0.8 o 3-δ perovskite oxide. Change the corresponding stoichiometric ratio, repeat the above steps, and use the same method to prepare SrTi 0.15 Nb 0.05 co 0.8 o 3-δ , SrTi ...
Embodiment 2
[0036] SrTi 0.1 Nb 0.1 co 0.8 o 3-δ Preparation and Oxygen Evolution Activity Evaluation of Perovskite Oxide
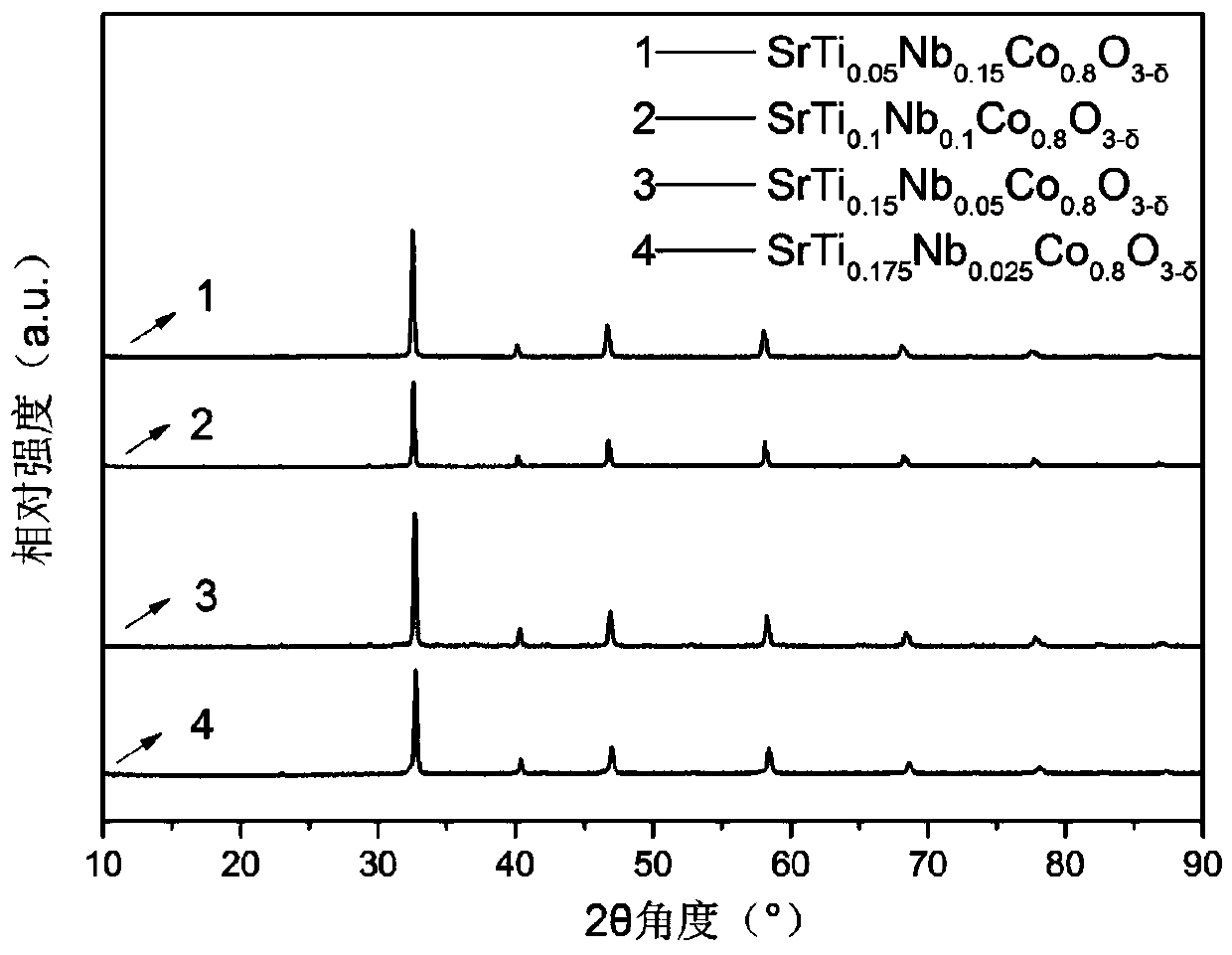
[0037] SrTi 0.1 Nb 0.1 co 0.8 o 3-δ The catalyst powder is prepared by a solid phase method. The specific steps are to combine SrCO with stoichiometric ratio 3 、TiO 2 , Nb 2 o 5 、Co 2 o 3 , Dispersed in ethanol solution, ball milled for 1h, after the solvent was evaporated, roasted. The calcination temperature is 1250°C, and the calcination time is 20h. Figure 7 The given X-ray diffraction (XRD) curve shows that the synthesized catalyst forms a perovskite structure.
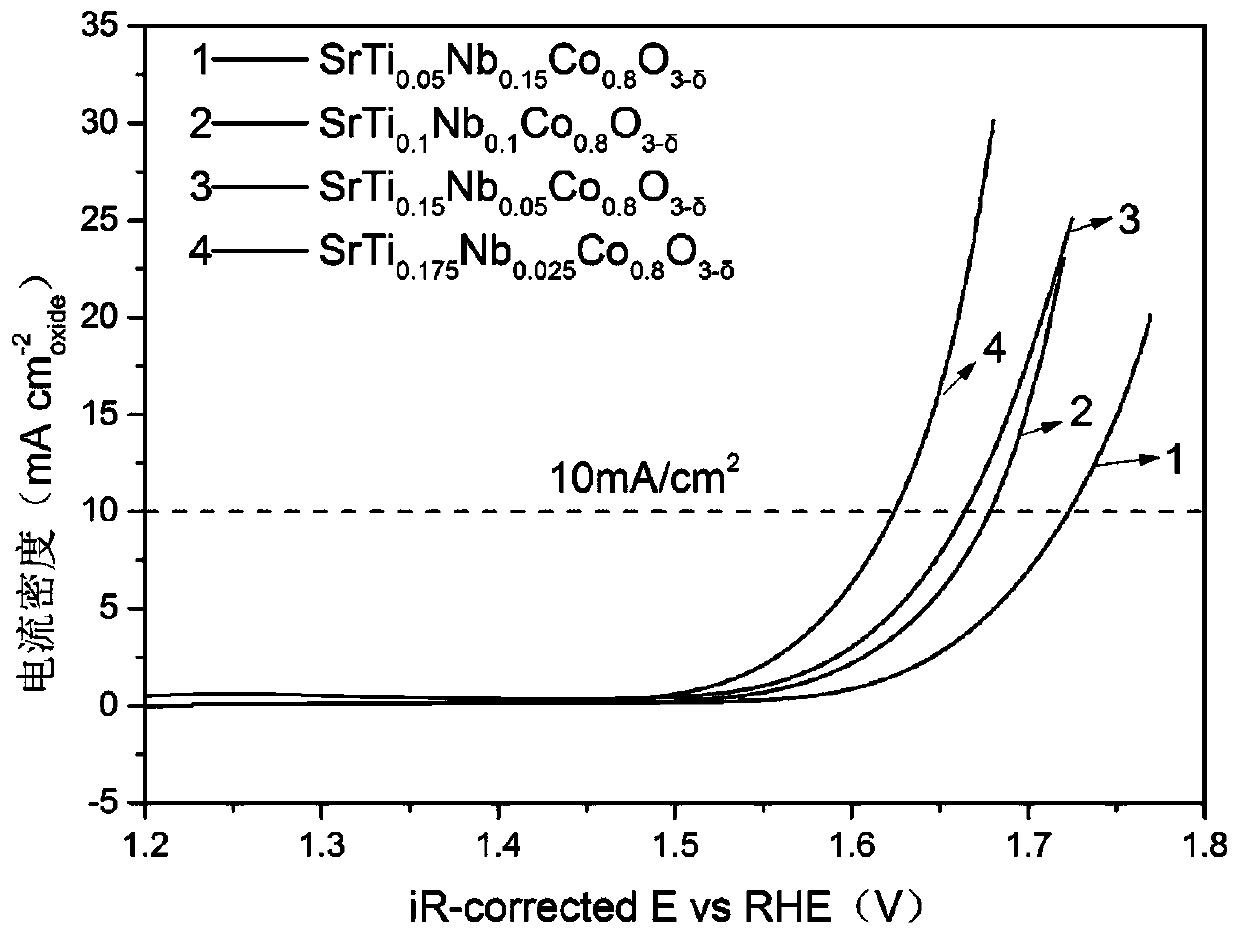
[0038] Oxygen evolution performance evaluation of catalysts. The electrode preparation and testing process are the same as in Example 1. Figure 8 The prepared SrTi is given 0.1 Nb 0.1 co 0.8 o 3-δ perovskite oxide in O 2 Polarization curve in saturated 0.1mol / L KOH solution.
Embodiment 3
[0040] SrTi 0.1 Nb 0.1 Fe 0.8 o 3-δ Preparation and Oxygen Evolution Activity Evaluation of Perovskite Oxide
[0041] SrTi 0.1 Nb 0.1 Fe 0.8 o 3-δ Perovskite oxides were prepared by a solid-state method, and the specific steps were stoichiometrically mixing SrCO 3 、TiO 2 , Nb 2 o 5 , Fe 2 o 3 , Dispersed in ethanol solution, ball milled for 1h, after the solvent was evaporated, roasted. The calcination temperature is 1250°C, and the calcination time is 20h. Synthesized according to stoichiometric ratio. Figure 9 X-ray diffraction (XRD) curves are given, showing that the synthesized catalyst forms a perovskite structure. Figure 10 The prepared SrTi is given 0.1 Nb 0.1 Fe 0.8 o 3-δ perovskite oxide in O 2 Polarization curve in saturated 0.1mol / L KOH solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com