Fluorescent material for detecting mustard gas mimics based on sulphur-pi interaction, and preparation method and application thereof
A fluorescent material and alkyl technology, applied in the preparation of organic compounds, dehydration of hydroxyl compounds to prepare ethers, luminescent materials, etc., can solve the problems of expensive instruments and complicated operations, and achieve the effect of low sensitivity and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
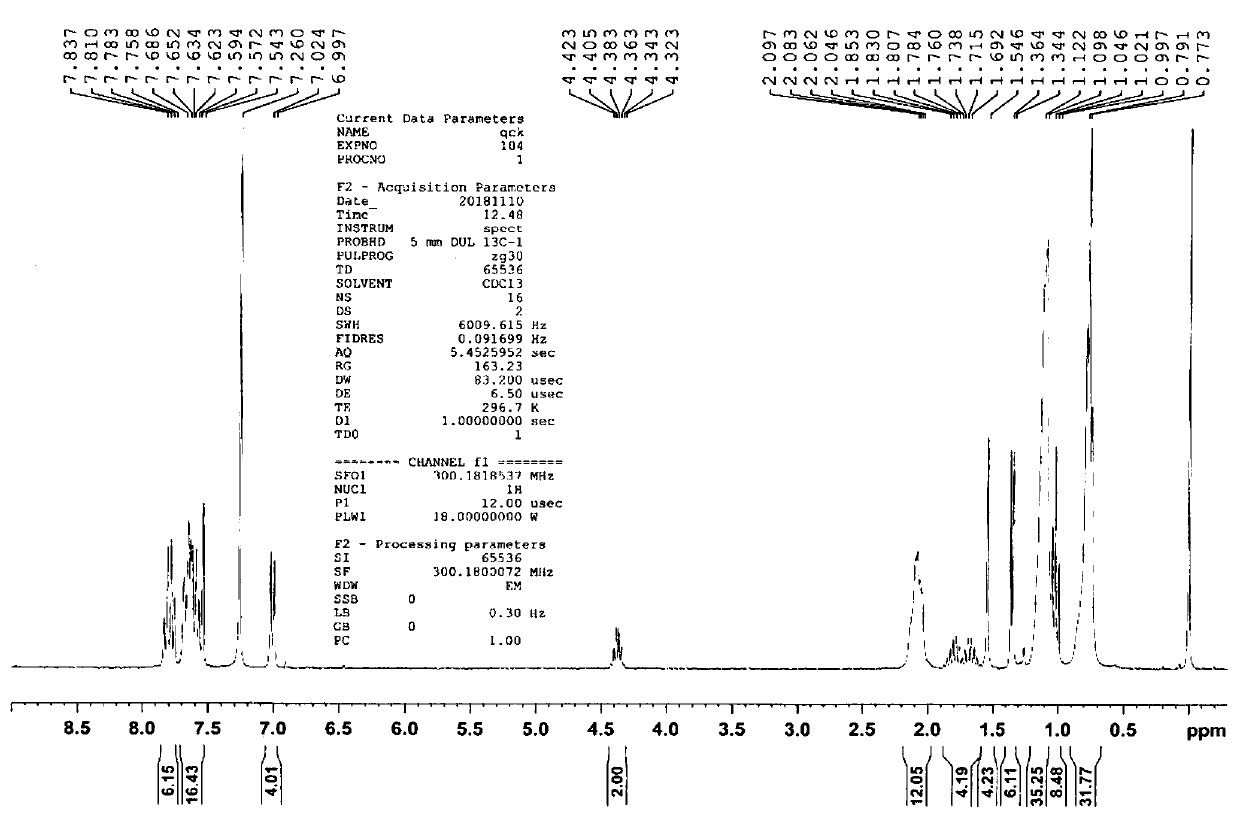
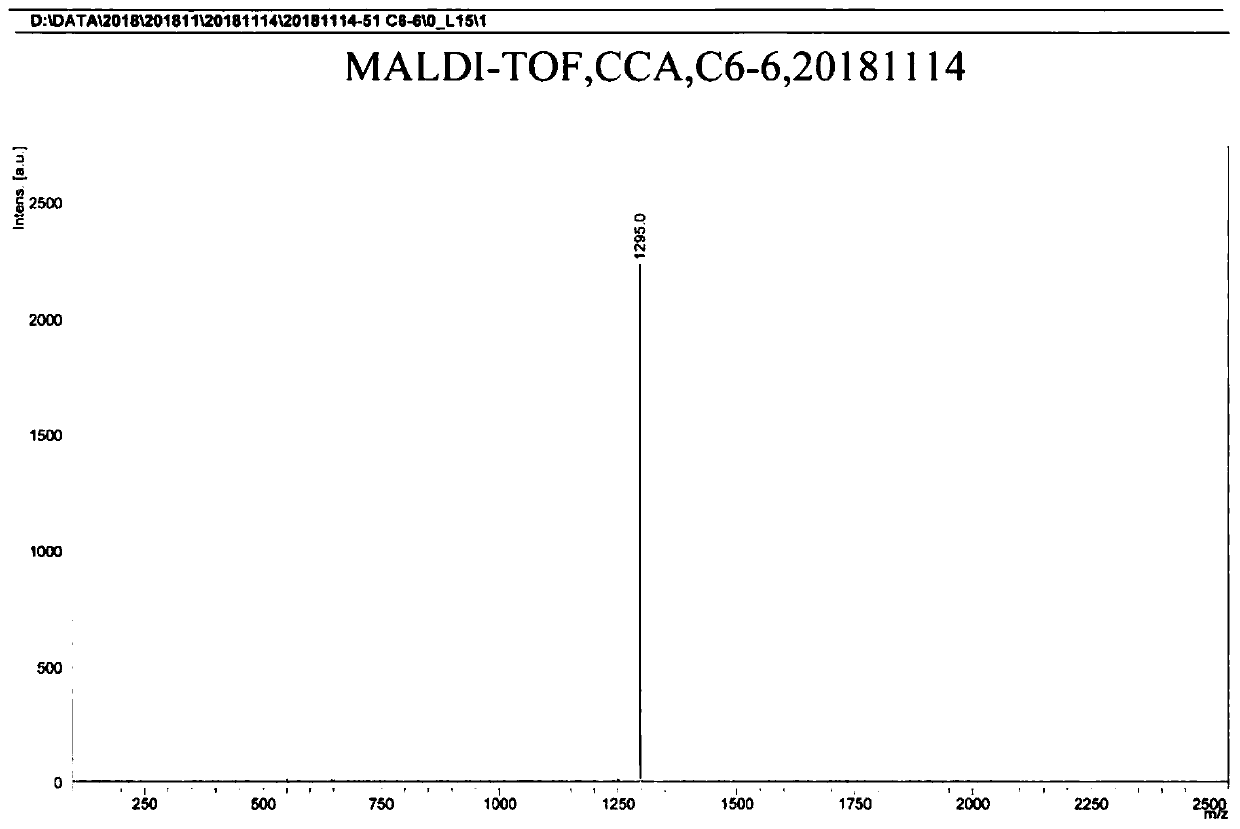
[0068] Preparation of compound 1
[0069]
[0070] (1) Add 2 g of 4-bromophenol, 1 g of 2-butanol, 30 ml of tetrahydrofuran, and 3.6 g of triphenylphosphine into a round bottom flask, add argon gas under stirring to deoxygenate for 10 minutes, and then place it in an ice water bath Under the conditions, slowly add 2.8 g of diisopropyl azodicarboxylate, return to normal temperature after dropping, and stir for 5 hours. The product obtained is obtained after separation by column chromatography;
[0071] (2) Take 1.7 grams of the product obtained in step (1) in a round bottom flask, add 2.3 grams of divaleryl diboron, 2.2 grams of potassium acetate, 0.3 grams of 1,1'-bis(diphenylphosphino) two Ferrocene palladium(II) dichloride, add 30 ml of 1,4-dioxane, add argon gas to remove oxygen under stirring conditions for 10 minutes, react at 80 degrees Celsius for 8 hours, the resulting product is separated by column chromatography get;
[0072] (3) Take 1.1 g of the product obtained in step...
Embodiment 2
[0084] Compound 2 with the following molecular structure was prepared.
[0085]
[0086] (1) Take 1.9 grams of 1-bromo-4-isopentyloxybenzene in a round bottom flask, add 2.8 grams of divaleryl diboron, 2.6 grams of potassium acetate, 0.5 grams of 1,1'-bis(diphenyl Phosphine) ferrocene palladium(II) dichloride, add 30 ml of 1,4-dioxane, add argon gas to remove oxygen under stirring conditions for 10 minutes, react at 80 degrees Celsius for 8 hours, the resulting product passes through the column Obtained after chromatographic separation;
[0087] (2) Take 1.45 grams of the product obtained in step (1), add 2.85 grams of 9,9-dioctyl-2,7-dibromofluorene, 0.6 grams of tetrakistriphenylphosphine palladium, and 2.3 grams of potassium carbonate to it. Add 30 ml of 1,4-dioxane and 6 ml of water, add argon gas to remove oxygen under stirring conditions for 10 minutes, react at 80 degrees Celsius for 8 hours, and obtain the product after separation by column chromatography;
[0088] (3) Take...
Embodiment 3
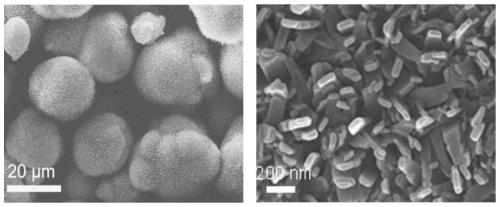
[0098] Using compound 1 obtained in Example 1, the microsphere aggregates prepared by self-assembly in a mixture of chloroform and methanol were used to detect mustard gas mimic 2-chloroethyl ethyl sulfide.
[0099] The microsphere aggregates prepared by self-assembly of compound 1 were coated in a self-made quartz tube, and then loaded into the testing equipment independently developed by the laboratory, and the 385 nanometer excitation light source was used to excite the microsphere aggregates. Use a 10 ml syringe to draw different concentrations of mustard gas simulant 2-chloroethyl ethyl sulfide vapor, and blow it into the quartz tube at a speed of 1 ml / sec. The detection result shows that the fluorescence will be quenched at 440nm. , The fluorescence enhancement phenomenon will occur at 540nm, and the detection sensitivity is high, the minimum concentration of 10ppb mustard gas simulant-2-chloroethyl ethyl sulfide can be detected, such as Image 6 Shown. The response time is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com