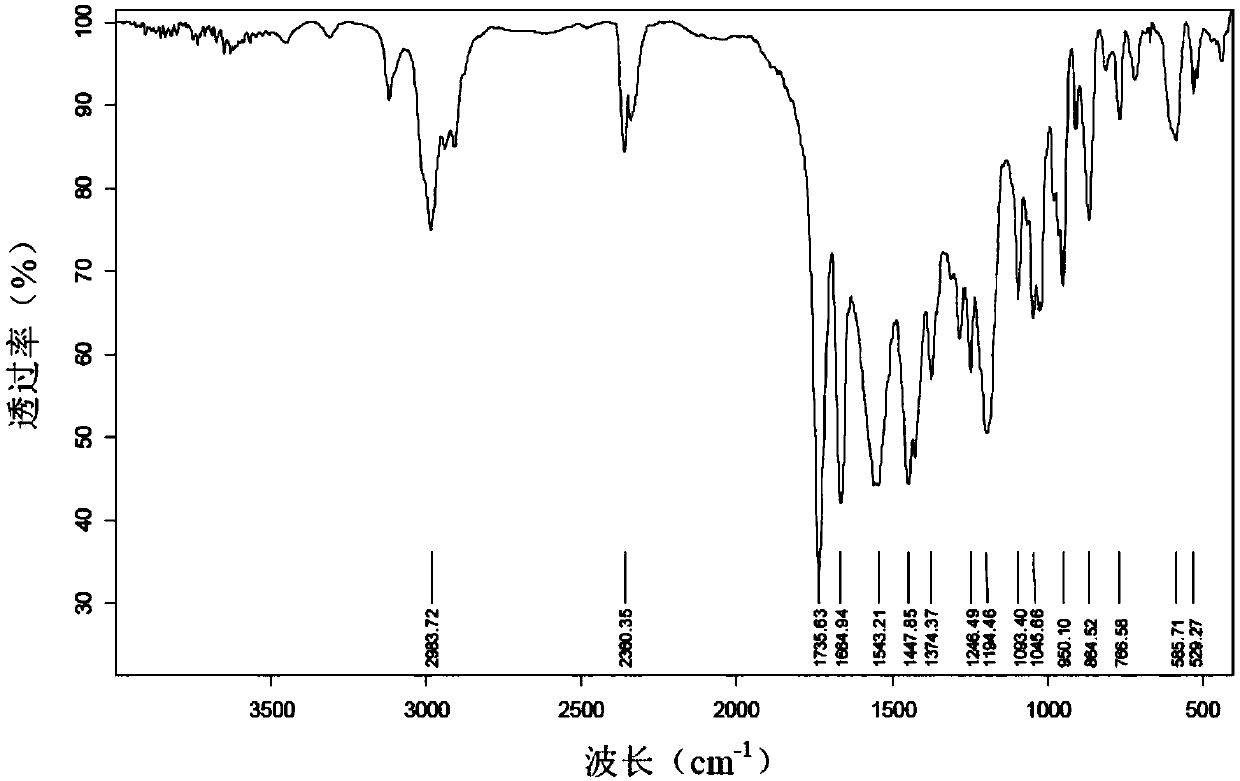
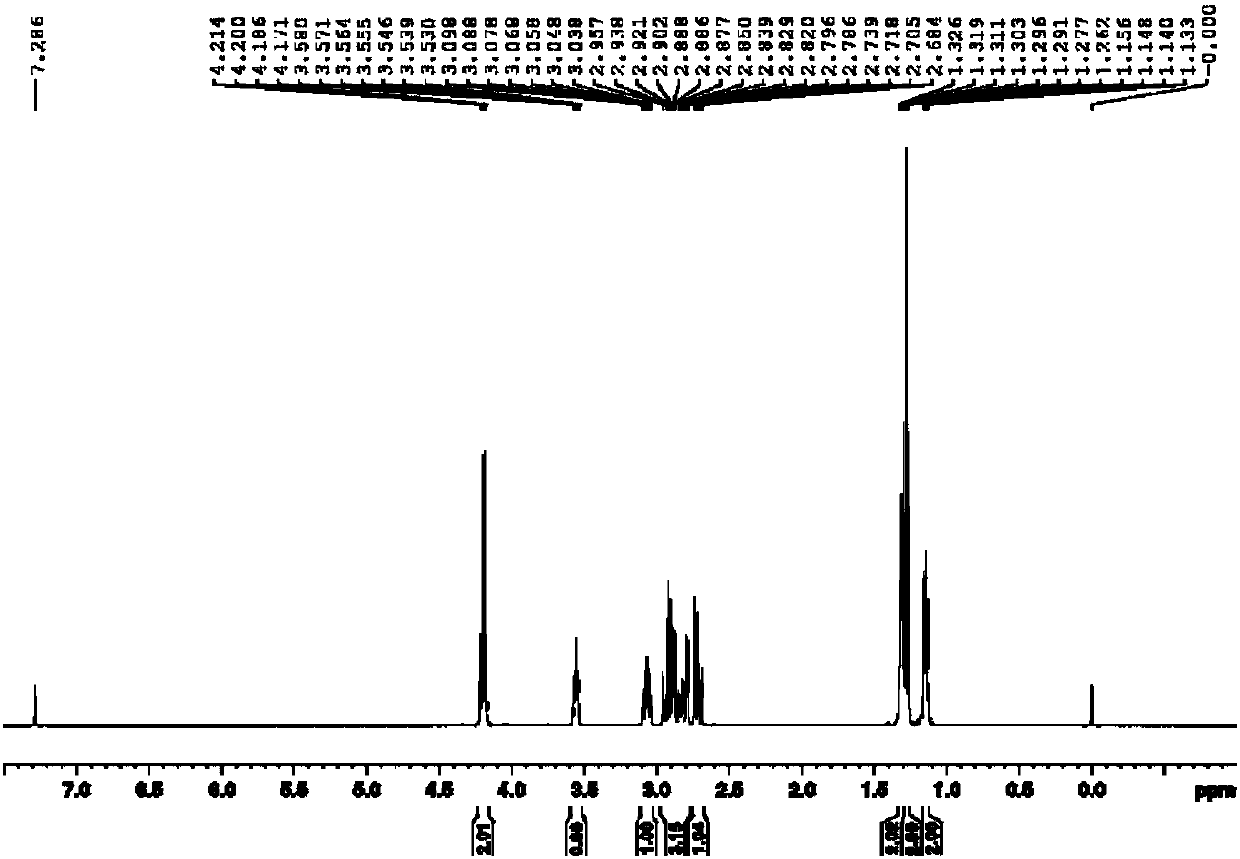
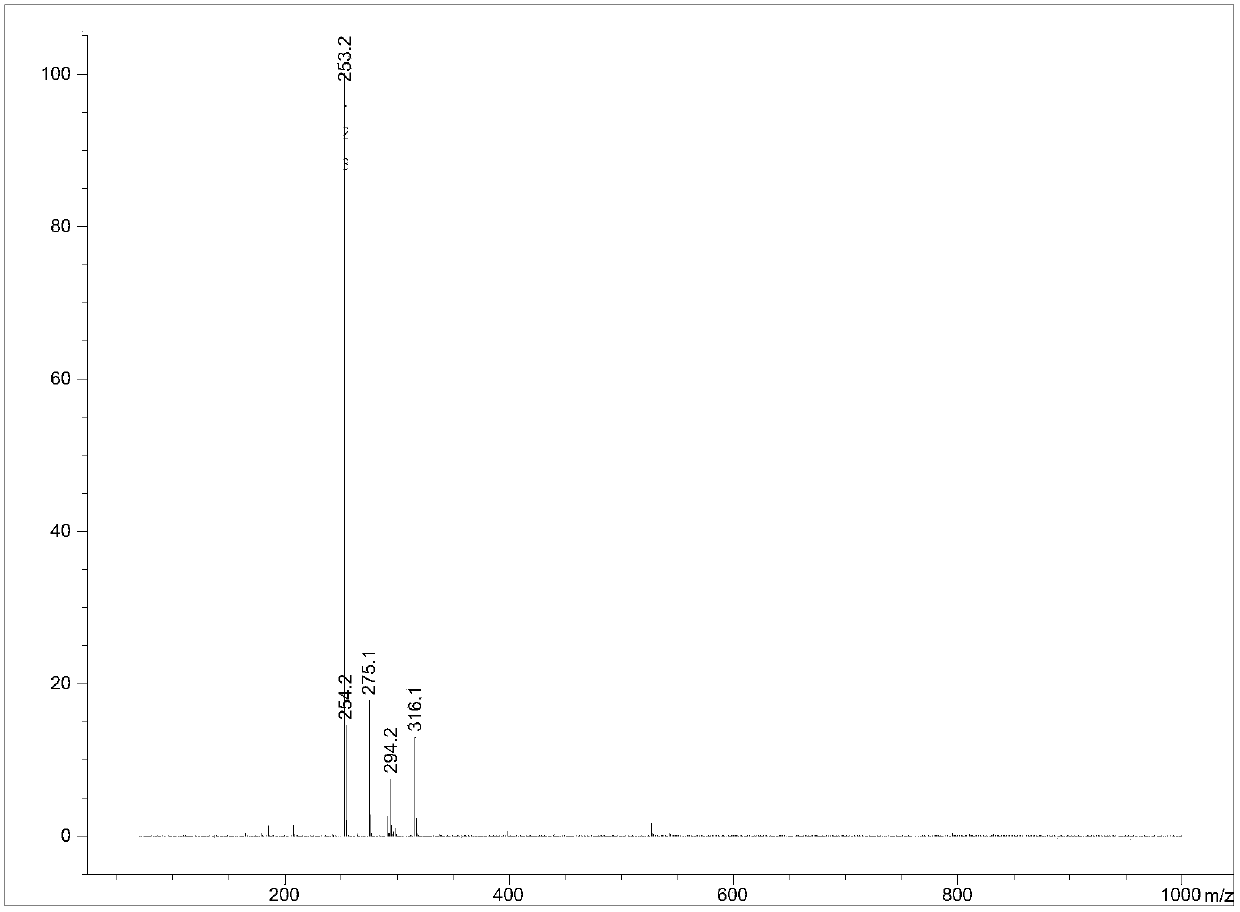
Production method of 4-cyclopropyl (hydroxy) methylene -3,5-diketone ethyl cyclohexane carboxylate and recycling method thereof
A technology of ethyl cyclohexanecarboxylate and a recovery method is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problems of reduced economic benefits, increased production costs, etc. The effect of improving economic efficiency and simple and easy recovery method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0058] According to a preferred embodiment of the present invention, the recovery method of described 4-cyclopropyl (hydroxyl) methylene-3,5-diketone cyclohexanecarboxylate ethyl ester comprises the following steps:
[0059] (1) under the condition of complexation reaction, will contain the liquid mixture of the compound shown in formula (1) and metal ion in the first organic solvent (can be a kind of in methanol, ethanol, Virahol and tert-butanol or multiple, preferably ethanol), and the contacted mixture is subjected to solid-liquid separation to obtain a solid phase and a liquid phase, and the obtained solid phase is washed and dried, wherein the metal is listed in column 9, column 1 of the periodic table of elements One or more of the metal elements in column 10, column 11, and column 12 (preferably, the metal ion is provided in the form of metal organic acid salt, and more preferably, the metal organic acid salt is copper acetate, acetic acid One or more of cobalt, nickel...
preparation example
[0139] This preparation example is used to illustrate the preparation method of the compound represented by the formula (2).
[0140] In a 3L stainless steel autoclave, 695 g (4.0 mol) of diethyl maleate (DM), 814 g (14 mol) of acetone and 73.5 g (1 mol) of diethylamine were added successively, and the nitrogen was replaced twice. The internal pressure was 0.55 MPa, and the reaction was maintained and pressured for 12 hours under this condition, and then the sample was sampled and detected by GC, and the content of DM was less than 1% by weight. Transfer the material to the distillation device, distill at atmospheric pressure, receive the distillate (acetone, diethylamine), when the kettle temperature rises to 60 ° C, change to negative pressure precipitation, the vacuum degree is -0.095MPa, collect 60 ~ 80 °C cut (transition cut). Then, high vacuum distillation was used with a vacuum degree of 6 mmHg, and 751 g of all fractions were collected, the purity of the compound (DOP...
Embodiment 1
[0143] Preparation of compound represented by formula (1):
[0144] (1) 1200g of toluene, 70g (1.5mol) of dehydrated alcohol and 225g (3.3mol) of solid sodium ethoxide were added successively in a 3L reaction flask, and the temperature was raised to 35° C. with stirring. The compound 690g (3.0mol) shown in formula (2) took 3 hours, and after the dropwise addition, the reaction was continued for 1 hour to obtain the ECOC reaction mixture;
[0145] (2) The ECOC reaction mixture obtained in step (1) was cooled to 20° C., and 76.5 g (0.75 mol) of triethylamine was added. After the triethylamine was completely dissolved, 345 g (3.3 g of cyclopropanecarbonyl chloride) was added dropwise to it. mol) was used for 2 hours, continued to react for 1 hour after the dropwise addition, then added tap water 800g, washed with water, left standstill, layered, separated the water layer, concentrated the toluene layer under negative pressure (-0.095MPa), and removed about 600g of toluene to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com