Synthesizing method of ambroxol hydrochloride related substance
A technology of ambroxol hydrochloride and related substances, applied in the field of organic synthesis, can solve the problems of the highest purity less than 95%, low purity of tetrahydroquinazoline, low synthesis yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
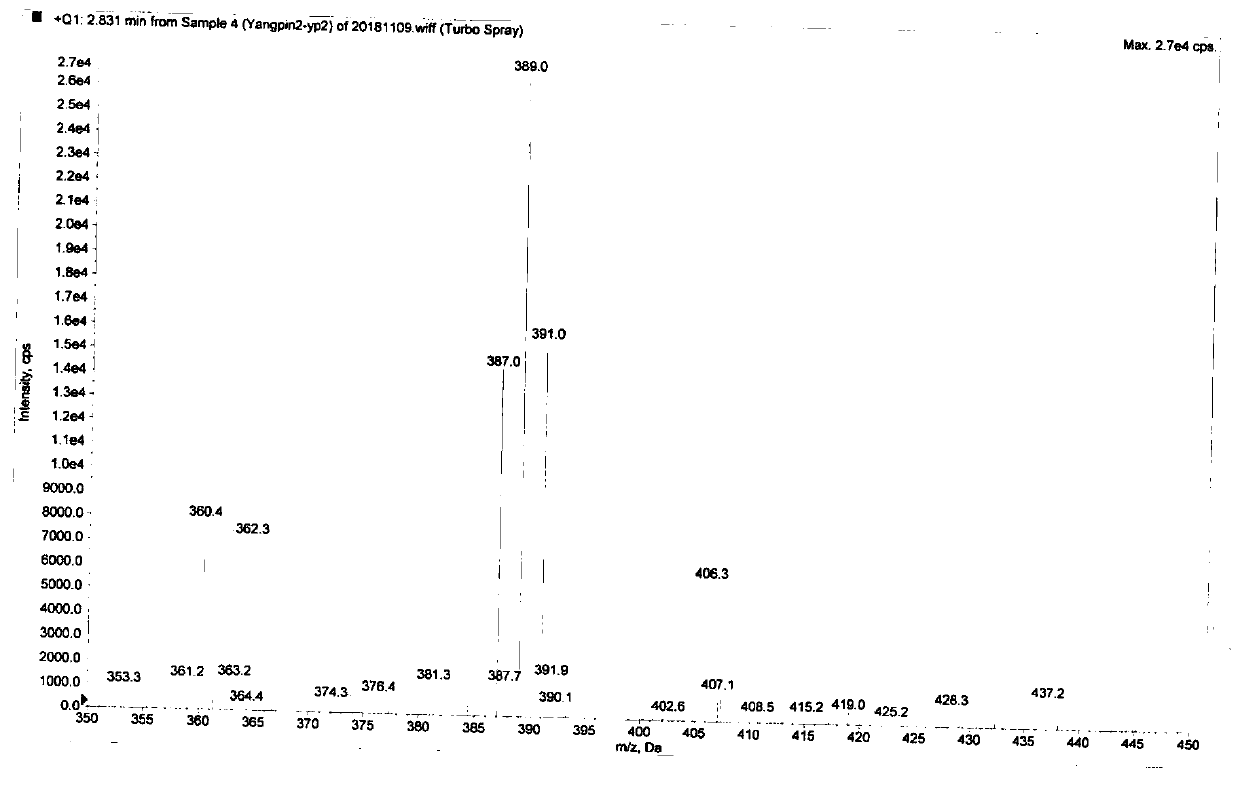
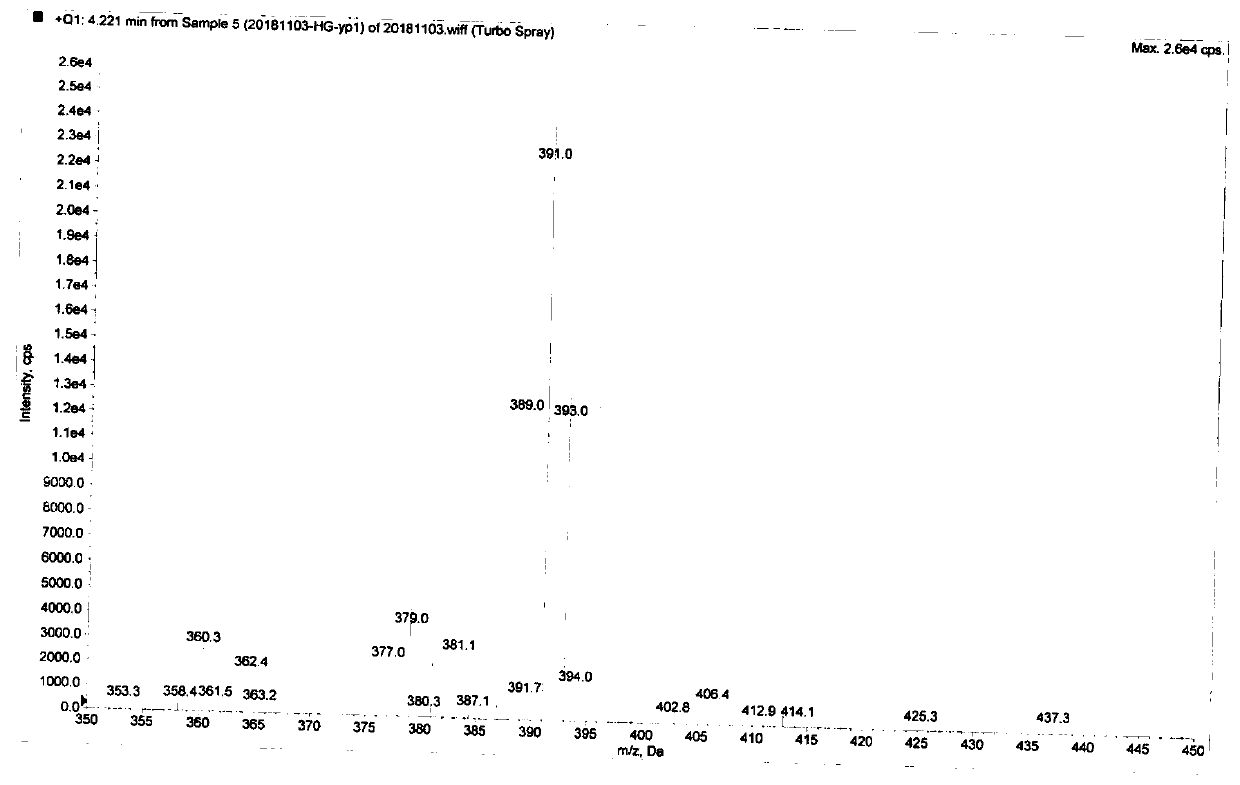
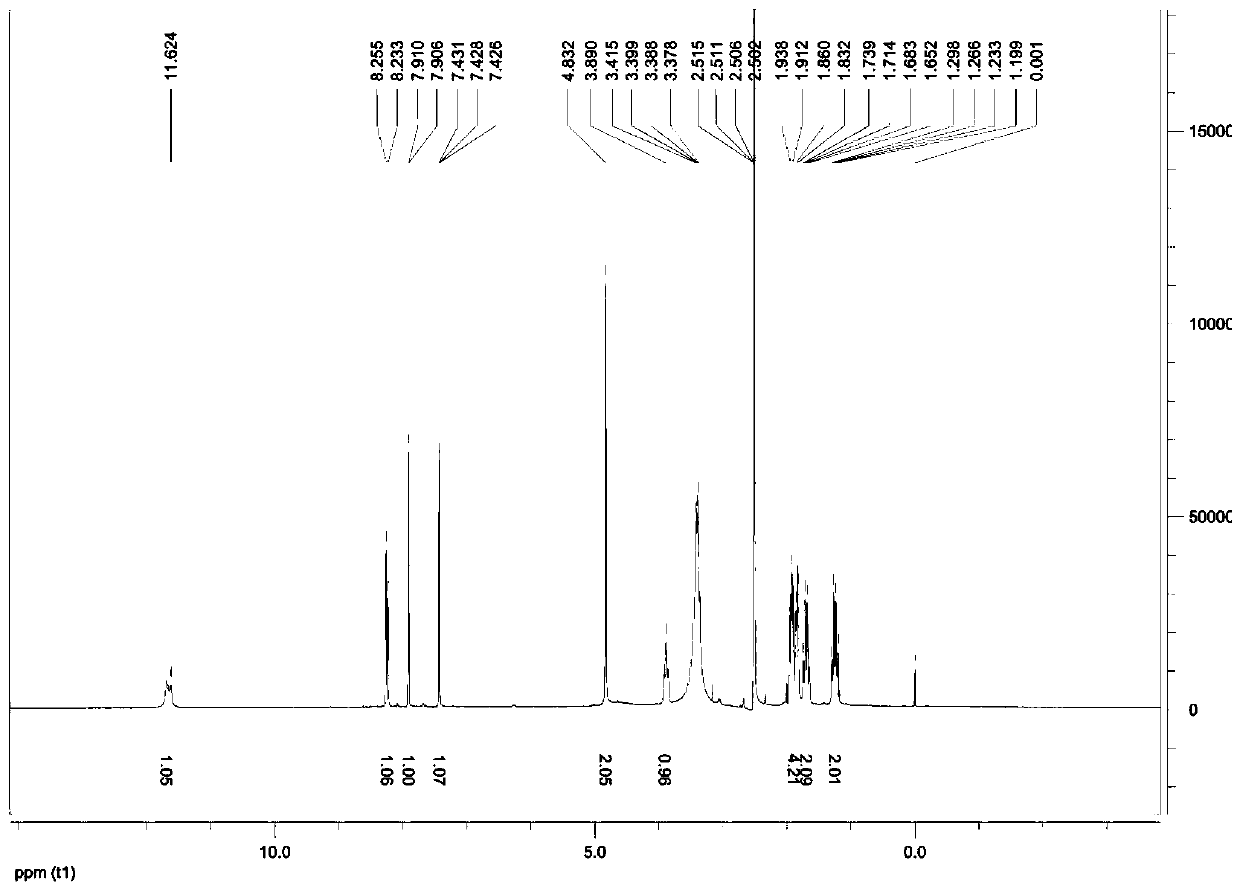
Image
Examples
Embodiment 1
[0031] 1. Synthesis of 4-(6,8-dibromo-1,4-dihydroquinazolin-3(2H)-yl)-cyclohexanol hydrochloride
[0032] Ambroxol hydrochloride (8.3g, 20mmol), methanol (60mL), and water (20mL) were successively added to a 250mL round-bottomed flask, and 37% formaldehyde aqueous solution (15mL) was slowly added, stirred at 25°C for 10h, and the reaction was detected by TLC . After the reaction, methanol was recovered under reduced pressure at 40°C, crystallized by cooling, suction filtered, washed with cold absolute ethanol, and dried at 50°C to obtain a white solid with a yield of 93% and a purity of 98.6%.
[0033] 2. Synthesis of 4-(6,8-dibromo-3,4-dihydroquinazolin-3-yl)-cyclohexanol hydrochloride
[0034] The product from the previous step (6g, 14mmol), dichloromethane (200mL), and cerium ammonium nitrate (19.2g, 35mmol) were successively added into a 250mL round bottom flask, stirred at room temperature for 10 h, and the reaction was detected by TLC. After the reaction, add water (10...
Embodiment 2
[0036] 1. Synthesis of 4-(6,8-dibromo-1,4-dihydroquinazolin-3(2H)-yl)-cyclohexanol hydrochloride
[0037] With embodiment 1.
[0038] 2. Synthesis of 4-(6,8-dibromo-3,4-dihydroquinazolin-3-yl)-cyclohexanol hydrochloride
[0039] The product from the previous step (6g, 14mmol), dichloromethane (200mL), and manganese dioxide (3g, 35mmol) were sequentially added into a 250mL round-bottomed flask, stirred at room temperature for 10h, and the reaction was detected by TLC. After the reaction, add water (100mL), separate the aqueous layer, adjust the pH to 7-8 with 5% sodium carbonate solution, extract with ethyl acetate (80mL*3), combine the organic layers, and wash the organic layer with water (50mL*3), without Dry over sodium sulfate, recover ethyl acetate under reduced pressure to 10 mL, add concentrated hydrochloric acid (1 mL) with stirring, cool and crystallize, filter with suction, wash with ethyl acetate, and dry at 50°C to obtain a white solid.
Embodiment 3
[0041]1. Synthesis of 4-(6,8-dibromo-1,4-dihydroquinazolin-3(2H)-yl)-cyclohexanol hydrochloride
[0042] With embodiment 1.
[0043] 2. Synthesis of 4-(6,8-dibromo-3,4-dihydroquinazolin-3-yl)-cyclohexanol hydrochloride
[0044] The product from the previous step (6 g, 14 mmol), dichloromethane (200 mL), and 10% palladium carbon (5 g) were successively added into a 250 mL round bottom flask, stirred at room temperature for 10 h, and the reaction was detected by TLC. Add water (100mL) after the reaction, separate the aqueous layer, adjust the pH to 7-8 with 5% sodium carbonate solution, extract with ethyl acetate (80mL*3), combine the organic layers, wash the organic layer with water (50mL*3), and remove Dry over sodium sulfate, recover ethyl acetate under reduced pressure to 10 mL, add concentrated hydrochloric acid (1 mL) under stirring, cool and crystallize, filter with suction, wash with ethyl acetate, and dry at 50°C to obtain a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com