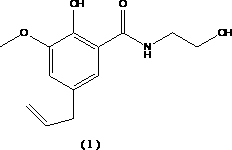
Choleretic drug alibendol preparation method
A technology of alibendol and choleretic drugs, which is applied in the field of drug synthesis, can solve the problems of harsh reaction conditions, low yield, and difficulty in realizing industrial production in the synthesis route, and achieve the effects of less pollution, simple synthesis steps, and alleviation of environmental problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
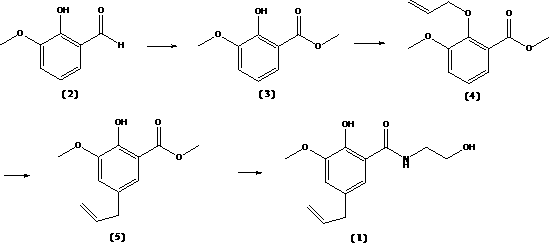
[0022] Preparation of methyl 2-hydroxy-3-methoxybenzoate (3): 7.6g of 2-hydroxy-3-methoxybenzaldehyde (2) and 1g of vanadium pentoxide were placed in a dry container with a reflux device In a three-neck round bottom flask, add 100ml of methanol, then add 30ml of 50% hydrogen peroxide, and stir at room temperature for 3 hours. After the reaction, filter and distill the filtrate to recover methanol, add 50ml of dichloromethane to wash twice, combine the dichloromethane phase, concentrate the solvent to dryness, and obtain 7.8g of a dark paste solid. The crude product was heated and recrystallized by adding 36ml of acetone and 0.5g of activated carbon to obtain 5.4g of methyl 2-hydroxy-3-methoxybenzoate as a white powder with a content of 93.5%.
[0023] Preparation of 2-hydroxy-3-methoxy-5-allylbenzoic acid methyl ester (5): under nitrogen protection, add 2-hydroxy-3-methoxybenzoic acid methyl ester ( 3) 10.0g, 7.2g of anhydrous potassium carbonate, add 100ml of acetone as a so...
Embodiment example 2
[0026] Preparation of methyl 2-hydroxy-3-methoxybenzoate (3): 7.6g of 2-hydroxy-3-methoxybenzaldehyde (2) and 1g of vanadium pentoxide were placed in a dry container with a reflux device In a three-neck round bottom flask, add 100ml of methanol, then add 50ml of 30% hydrogen peroxide, and stir at room temperature for 8 hours. After the reaction, filter and distill the filtrate to recover methanol, add 50ml of dichloromethane to wash twice, combine the dichloromethane phase, concentrate the solvent to dryness, and obtain 8.2g of dark paste solid. The crude product was heated and recrystallized by adding 36ml of acetone and 0.5g of activated carbon to obtain 5.8g of white powder methyl 2-hydroxy-3-methoxybenzoate with a content of 94.7%.
[0027] Preparation of 2-hydroxy-3-methoxy-5-allylbenzoic acid methyl ester (5): under nitrogen protection, add 2-hydroxy-3-methoxybenzoic acid methyl ester ( 3) 10.0g, 4.2g of sodium hydroxide, add 100ml of acetone as a solvent, raise the tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com