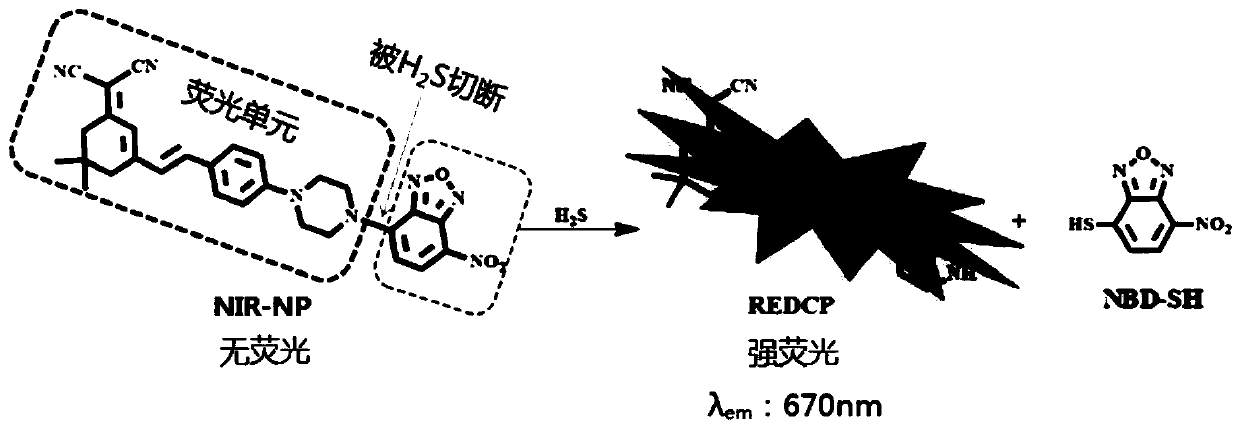
Near-infrared fluorescent probe for identifying hydrogen sulfide as well as preparation method and application thereof
A fluorescent probe, near-infrared technology, used in chemical instruments and methods, preparations for in vivo experiments, fluorescence/phosphorescence, etc., can solve the problems of sensitivity and accuracy of fluorescence analysis, and achieve good biological tissue permeability, Highly selective, less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] This example is used to illustrate the preparation of the fluorescent probe of the present invention.
[0050] The reaction formula is as follows:
[0051]
[0052] The preparation steps are as follows:
[0053] 1) Preparation of REDCP: 2-(3,5,5-trimethylcyclohex-2-enyl)malononitrile (100mg, 0.54mmol) and 4-(piperazin-1-yl)benzaldehyde ( 102mg, 0.54mmol) was dissolved in absolute ethanol (15ml). Then, five drops of piperidine were added to the solution under the protection of argon. After heating to reflux for 5 hours, the mixture solution was cooled to room temperature, and the solvent was removed under reduced pressure. The crude product was purified by silica gel column chromatography with dichloromethane / methanol (200:1) as eluent to give red solid REDCP (119 mg, 0.33 mmol) in 61% yield.
[0054] 2) Preparation of NIR-NP: under the protection of argon, 0.1mL N,N-diisopropyl-ethylamine (DIPEA) was added to 15ml of REDCP in anhydrous dichloromethane solution (9...
Embodiment 2
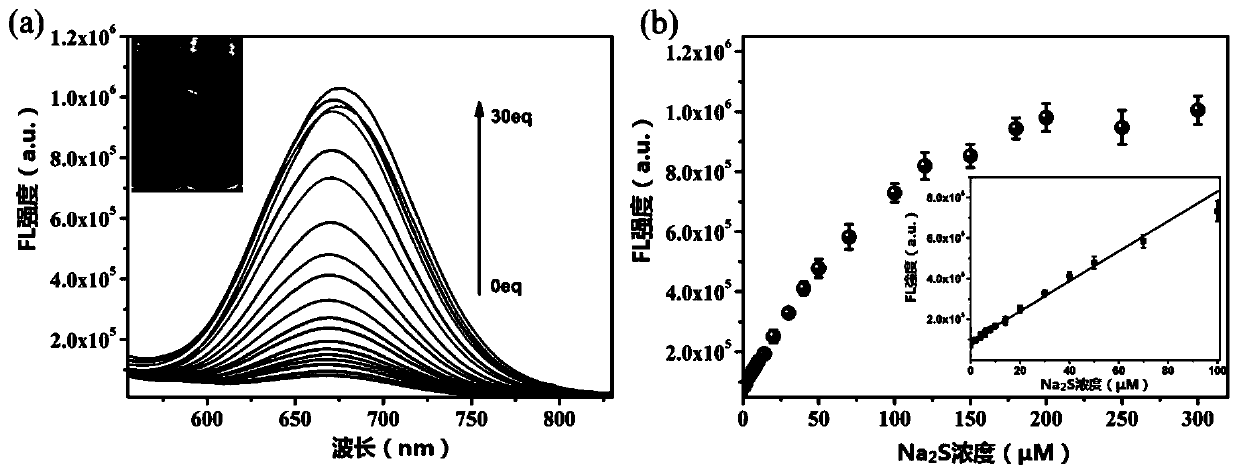
[0057] This example is used to illustrate that the fluorescence of NIR-NP varies with Na 2 Positive correlation change of S equivalent.
[0058] Different equivalents of Na 2 S was added to the probe NIR-NP solution and incubated at 37°C for 1 hour, excited with a 440nm laser, and its emission spectrum was recorded. Such as figure 2 (a) and figure 2 As shown in (b), in the absence of Na 2 In the case of S, almost no fluorescence is observed because the strong electron-pulling effect of NBD quenches the fluorescence. Added different concentrations of Na 2 After S, obvious fluorescence can be observed at 670nm, and the fluorescence intensity increases with Na 2 increases with the increase of S. figure 2 (a) and figure 2 (b) shows Na in the range of 0-100μM 2 Good linear relationship between S concentration and fluorescence intensity. Through calculation, the detection limit of this probe can be obtained as 0.03 μM, which is much lower than that of H in mammalian se...
Embodiment 3
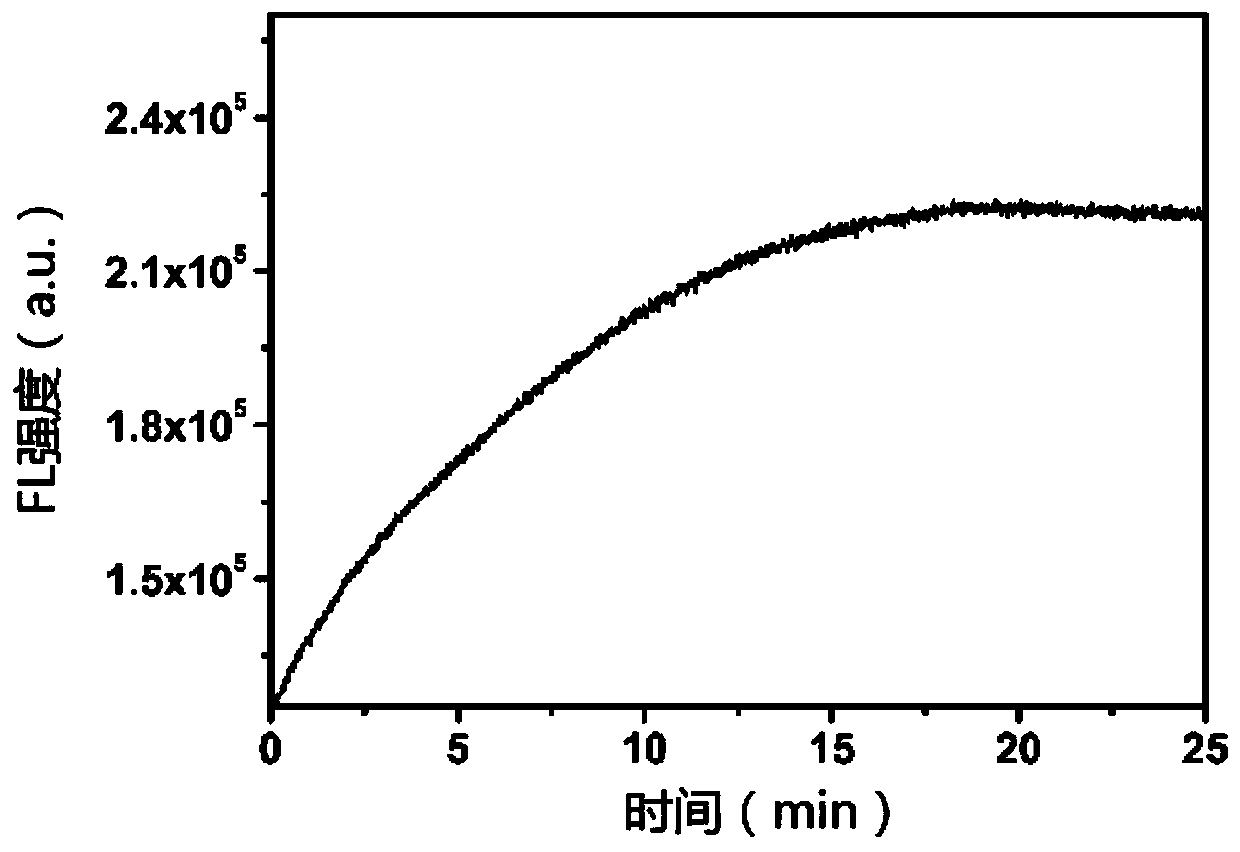
[0060] This example is used to illustrate that NIR-NP and Na 2 The change of fluorescence intensity with time after S reaction.
[0061] Add 100 μM Na to 10 μM NIR-NP solution 2 S, The fluorescence intensity of the probe was recorded with a fluorometer as a function of time. Such as image 3 As shown, the fluorescence intensity increased significantly and reached a maximum within 15 minutes of incubation. This result indicates that the probe can rapidly react with hydrogen sulfide in a short time, thereby quantitatively measuring hydrogen sulfide and dynamically monitoring the real-time production of endogenous hydrogen sulfide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com