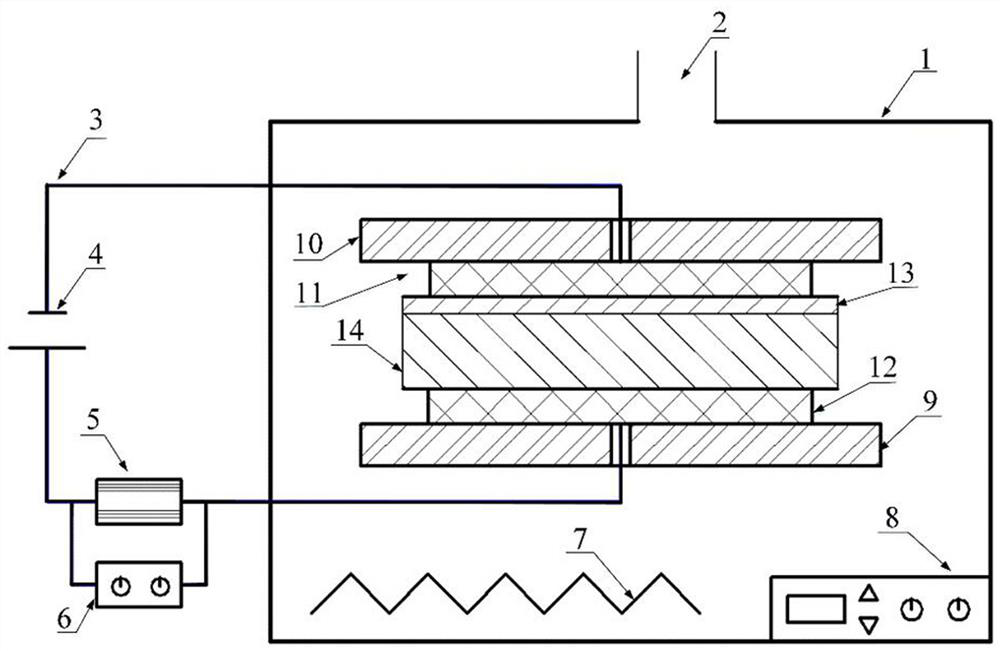
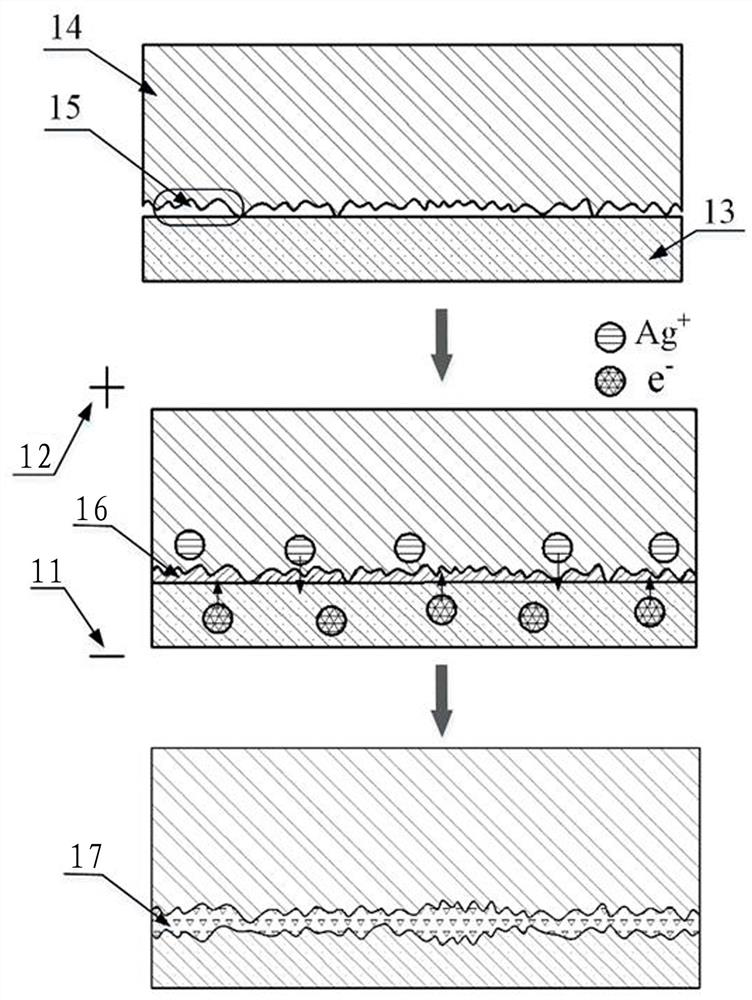
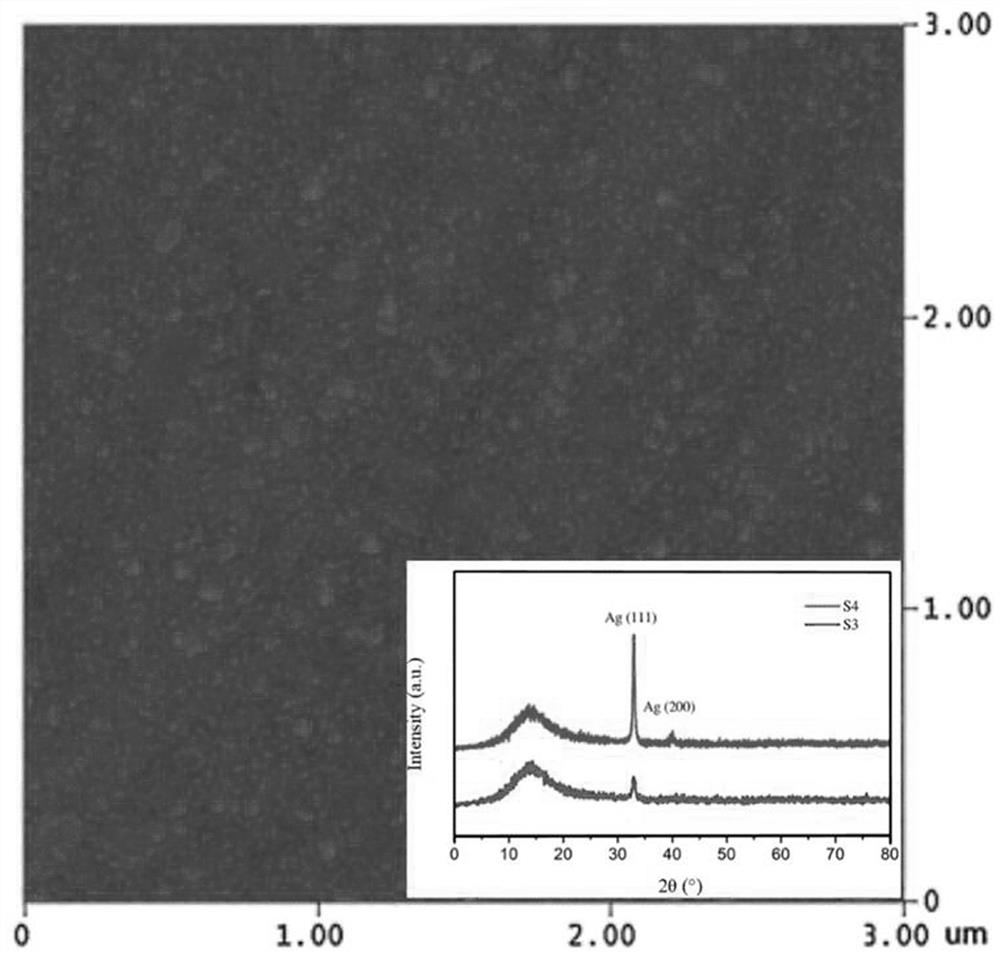
Surface in-situ metallization method based on cationic conductive glass
A conductive glass and cationic technology, applied in the field of surface metallization, can solve the problems of high bonding temperature, poor electrical and thermal conductivity of the bonding area, difficulty in adapting to high-power and high-integration device packaging requirements, etc., to reduce difficulty and reduce residual thermal stress , Good effect of promoting application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A surface in-situ metallization method based on cationic conductive glass, which is used to assist the brazing or bonding surface metal layer of cationic conductive glass, which is formed by the cationic reaction in the cationic conductive glass after being activated by the recombination field, without surface coating , sputtering or ion implantation, and proceed as follows:
[0030] Step 1: Select AgⅠ-Ag with high conductivity and low ionization activation energy 2 O-B 2 o 3 The silver ion conductive glass is used as the in-situ metallization sample on the surface. The preparation process is as follows: prepare chemically pure grade AgⅠ, Ag 2 O and B 2 o 3 As a raw material, the material is 60mol%AgI, Ag 2 O / B 2 o 3 The ratio of =3 is fully mixed, placed in a quartz glass tube with one end open, heated in an electric furnace to 480~800°C to melt, and then the melt is cooled by double rollers, during which the pressure of the double rollers is adjusted reasonably...
Embodiment 2
[0036] A surface in-situ metallization method based on cationic conductive glass, which is used to assist the brazing or bonding surface metal layer of cationic conductive glass, which is formed by the cationic reaction in the cationic conductive glass after being activated by the recombination field, without surface coating , sputtering or ion implantation, and proceed as follows:
[0037] Step 1: Select AgⅠ-Ag with high conductivity and low ionization activation energy 2 O-B 2 o 3 The silver ion conductive glass is used as the in-situ metallization sample on the surface. The preparation process is as follows: prepare chemically pure grade AgⅠ, Ag 2 O and V 2 o 5 As a raw material, the material is 50mol%AgI, Ag 2 O / V 2 o 5 The ratio of =0.67:0.33 is fully mixed, weigh 5g of mixed powder in a 250mL agate ball mill jar, put 10 agate grinding balls with a diameter of 10mm, and add 10mL of acetone as a process control agent, and use it in a planetary ball mill at a speed o...
Embodiment 3
[0043] A surface in-situ metallization method based on cationic conductive glass, which is used to assist the brazing or bonding surface metal layer of cationic conductive glass, which is formed by the cationic reaction in the cationic conductive glass after being activated by the recombination field, without surface coating , sputtering or ion implantation, and proceed as follows:
[0044] Step 1: Select Cu with high conductivity and low ionization activation energy 2 O-Al 2 o 3 -SiO 2 Copper ion conductive glass is used as the surface in-situ metallization sample, and the preparation process is as follows: according to 12.5%CuO-12.5%Al 2 o 3 -75%SiO2 2 The molar ratio of the ball was ground at a speed of 400rpm for 2h, and put into Al 2 o 3 Heat the melt in a crucible to 1550°C to melt, and then cool the melt through double rollers, during which the pressure of the double rollers is adjusted reasonably to control the cooling rate of the melt to obtain thin flake glass...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface roughness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com