Tri-hydroxy containing hexaquaternary phosphonium cation antibacterial agent and preparation method thereof
A technology of trihydroxyl and antibacterial agents, applied in chemical instruments and methods, disinfectants, organic chemistry, etc., can solve the problems that do not involve trihydroxyhexaquaternary phosphonium salt antibacterial agents, and achieve enhanced electrostatic interaction and high unit positive charge , to avoid the effect of loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
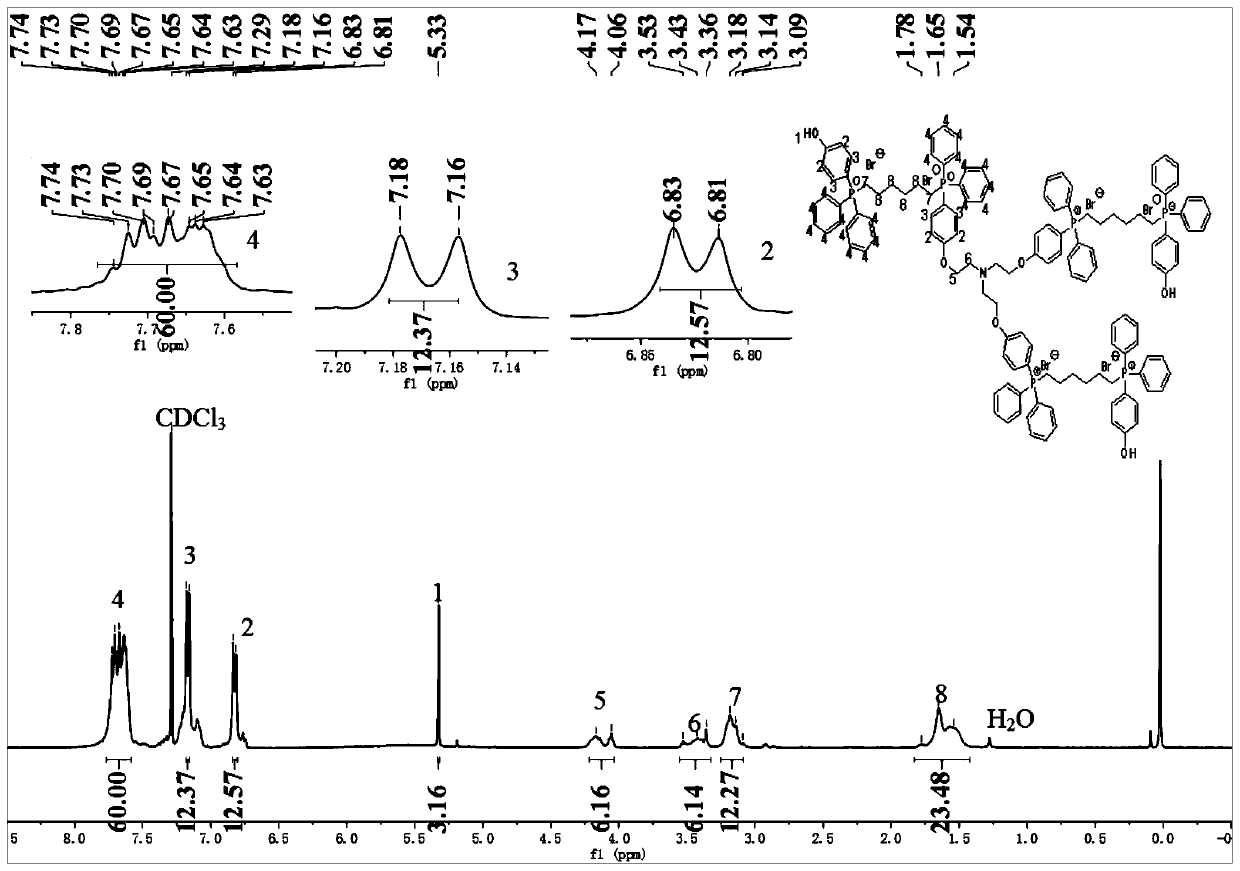
[0032] 8mmol of compound A and 2mmol of B were added to a 100mL three-necked flask, 30mL of methanol was added thereto, and 8mmol of NaOH was added. Reflux at 60°C and monitor the progress of the reaction by TLC spot plate. After the reaction is complete, cool to room temperature, remove solvent methanol by rotary evaporation, dissolve the crude product in dichloromethane, wash with deionized water, dry with anhydrous sodium sulfate, and remove dichloromethane by rotary evaporation. A pale yellow crude product was obtained. Further purification was carried out with a chromatographic column (petroleum ether and ethyl acetate).
[0033] Dissolve the purified product in dichloromethane, and then dropwise add 3mol L -1 HCl solution, monitor the progress of the reaction by TLC spot plate, after the reaction is complete, the organic phase is washed with deionized water, and the pH is adjusted until the solution is neutral. The dichloromethane was removed by rotary evaporation, an...
Embodiment 2
[0035] Add 8mmol of compound A and 2mmol of C to a 100mL three-necked flask, add 30mL of methanol, and then add 8mmol of NaOH. Reflux at 60°C and monitor the progress of the reaction by TLC spot plate. After the reaction is complete, cool to room temperature, remove solvent methanol by rotary evaporation, dissolve the crude product in dichloromethane, wash with deionized water, dry with anhydrous sodium sulfate, and remove dichloromethane by rotary evaporation. A pale yellow crude product was obtained. Further purification was carried out with a chromatographic column (petroleum ether and ethyl acetate).
[0036] Dissolve the purified product in dichloromethane, and then dropwise add 3mol L -1 HCl solution, monitor the progress of the reaction by TLC spot plate, after the reaction is complete, the organic phase is washed with deionized water, and the pH is adjusted until the solution is neutral. The dichloromethane was removed by rotary evaporation, and the light yellow pro...
Embodiment 3
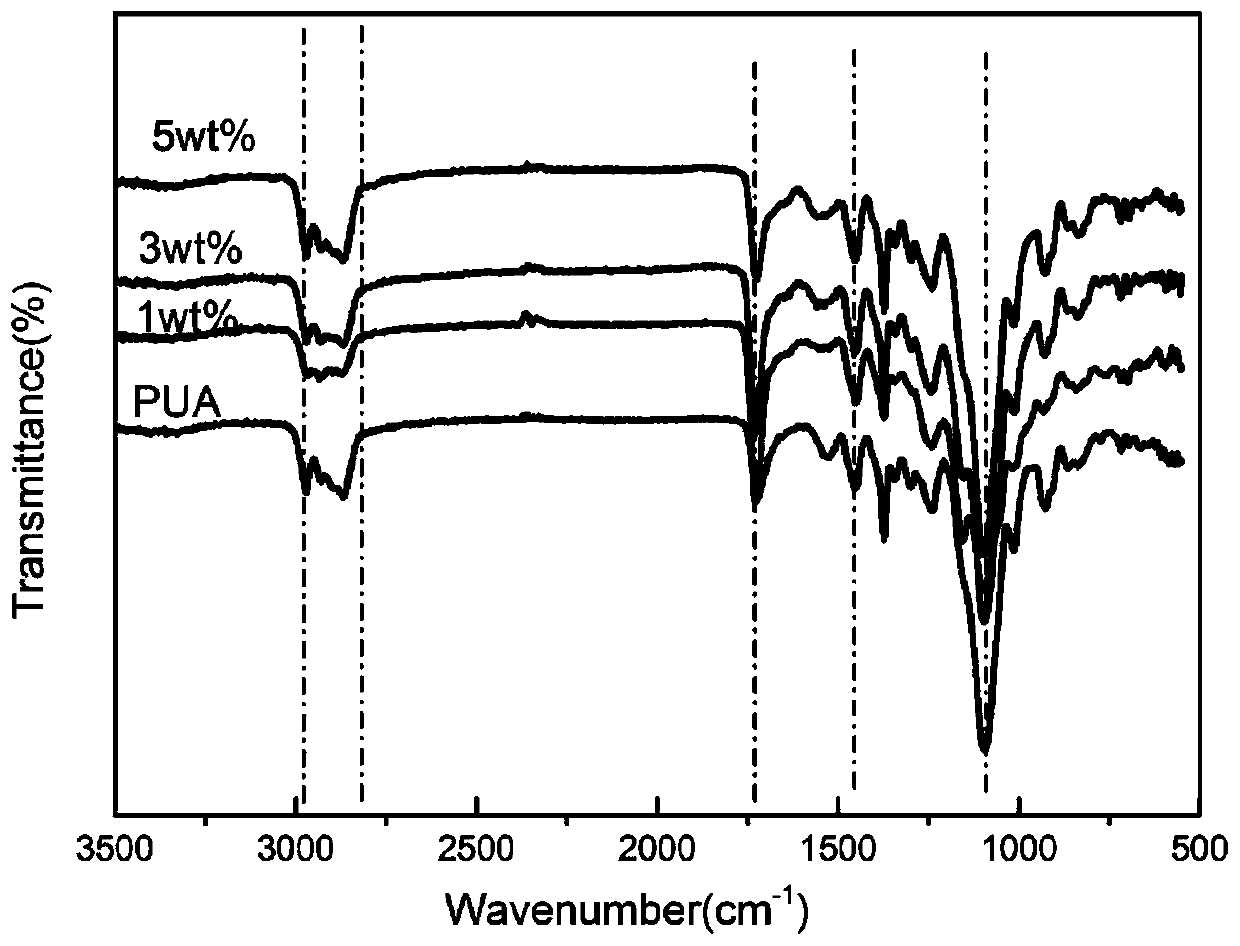
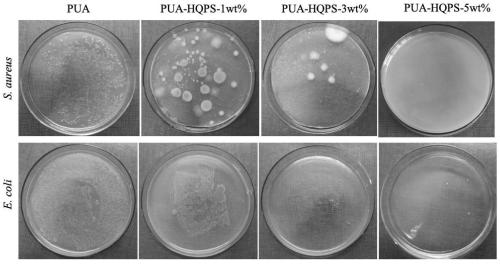
[0038]The trihydroxy hexaquaternary phosphonium salt (1wt%) obtained in Example 1, isophorone diisocyanate (1.04g) was added in a 50mL three-necked flask, and then mechanically stirred for 30 minutes at 50°C, and then 50mg was added to the bottle The catalyst is dibutyltin dilaurate, and then polyether polyol HSH330N is added dropwise at 65°C, and the stirring reaction is continued for 1 hour, then the stirring is stopped and cooled. After the system was cooled down to 45°C, 2-hydroxyethyl acrylate (HEA) was added dropwise into the system with a constant pressure dropping funnel to block the -NCO in the system. After reacting for 3 hours, the urethane acrylate / hexaquaternary phosphonium salt-1wt% oligomer was obtained.
[0039] Prepared urethane acrylate / hexaquaternary phosphonium salt-1wt% oligomer with reactive diluent (1,6-hexanediol diacrylate trimethylolpropane triacrylate, isobornyl acrylate) and photoinitiator 2 -Hydroxy-2-methyl-1-phenyl-1-propanone was added to a bea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com