Zika virus vaccine and preparation method thereof
A Zika virus and vaccine technology, applied in the field of virus vaccines, can solve the problems of DNA vaccine safety, RNA vaccine stability and application limitation, cross-linking virus structure damage, complicated preparation process, etc., so as to retain antigenicity and immunogenicity , good antigenicity and immunogenicity, the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
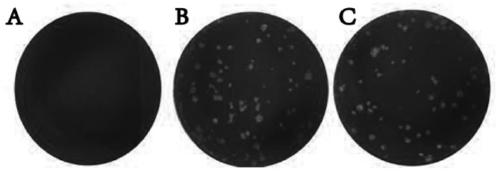
[0062] Example 2 Plaque method to detect infectivity of Zika virus inactivated by polypeptide Z2
[0063] In order to further detect whether the Z2 inactivated ZIKV can continue to infect cells and proliferate in cells, preferably, a plaque formation assay is used to detect the infectivity of the inactivated virus.
[0064] (1) 8×10 3 PFU Zika virus was mixed with 50 μM Z2, and incubated in a 37°C incubator for 24 hours;
[0065] (2) Simultaneously set ZIKV treated with DMSO and PBS as a control, and incubated in a 37°C incubator for 24h;
[0066] (3) ZIKV with different treatments was diluted 10 times to reduce the concentration of the polypeptide, and 1ml of the diluted solution was taken to infect the monolayer BHK-21 cells spread in a 6-well plate, at 37°C, 5% CO 2 Under the condition of adsorption for 2h;
[0067] (4) Discard the supernatant and replace it with DMEM medium containing 1% ultrapure low-melting point agarose (ThermoFisher, America) and 2% FBS;
[0068] (...
Embodiment 3
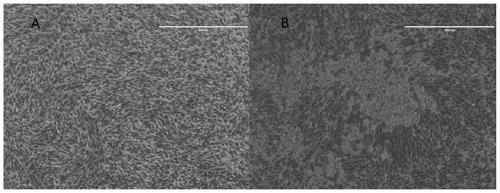
[0072] The distribution of embodiment 3 polypeptide Z2 on virus surface
[0073] In order to detect the direct interaction between Z2 and ZIKV, as well as its mode of action, preferably, stimulated emission depletion fluorescence microscopy (STED) is used to observe the interaction between Z2 and virus particles and the distribution state of Z2. In order to carry out fluorescent labeling to the virus, preferably, a general labeling method for enveloped viruses is adopted in the present invention, and biotin-labeled phosphatidylethanolamine (Biotin Cap PE, Avanti, America) is added to the cell culture medium to make the outer membrane of the cells and the inner membrane system are biotin-labeled; then use the labeled cells to culture ZIKV, and use the process of virus budding from the endoplasmic reticulum to obtain the envelope to label the virus with biotin; then use Cy5-labeled streptavidin (SA-Cy3 , ThermoFisher, America) Cy3-labeled ZIKV. During synthesis, polypeptide Z2 ...
Embodiment 4
[0083] The preparation of embodiment 4 vaccine
[0084] Through research on the mechanism of Z2 inactivation of ZIKV, the inventors found that ZIKV after Z2 inactivation releases genomic RNA, but retains the natural conformation of the viral envelope protein, making the inactivated ZIKV structurally similar to virus-like particles. The method for preparing Zika virus inactivated vaccine by using Z2 inactivated ZIKV will be further elaborated below in conjunction with specific examples.
[0085] Preparation of virus stock solution
[0086] (1) The day before inoculation, the Vero-E6 cells were subcultured so that they could grow to a monolayer the next day;
[0087] (2) Discard the cell supernatant, replace it with serum-free DMEM medium, add ZIKV stock solution, mix gently, and place at 37°C, 5% CO 2 Adsorption in the incubator for 2h;
[0088] (3) Add DMEM virus maintenance solution containing 2% FBS, place at 37°C, 5% CO 2 Cultivate in an incubator for 5-6 days;
[0089] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com