Indole hemicyanine fluorescent probe, preparation method and application to detection of cyanide ions
A fluorescent probe and indole hemicyanine technology, applied in the field of chemical synthesis and analysis and detection, can solve the problems of time-consuming, expensive equipment, high detection limit, etc., and achieve the effect of high yield, rapid reaction and short synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of 2-(2-(2-chloro-7-diethylaminoquinolin-3-yl)vinyl-N,3,3-trimethyl-3H-indole (QIE) (stirred reflux method).
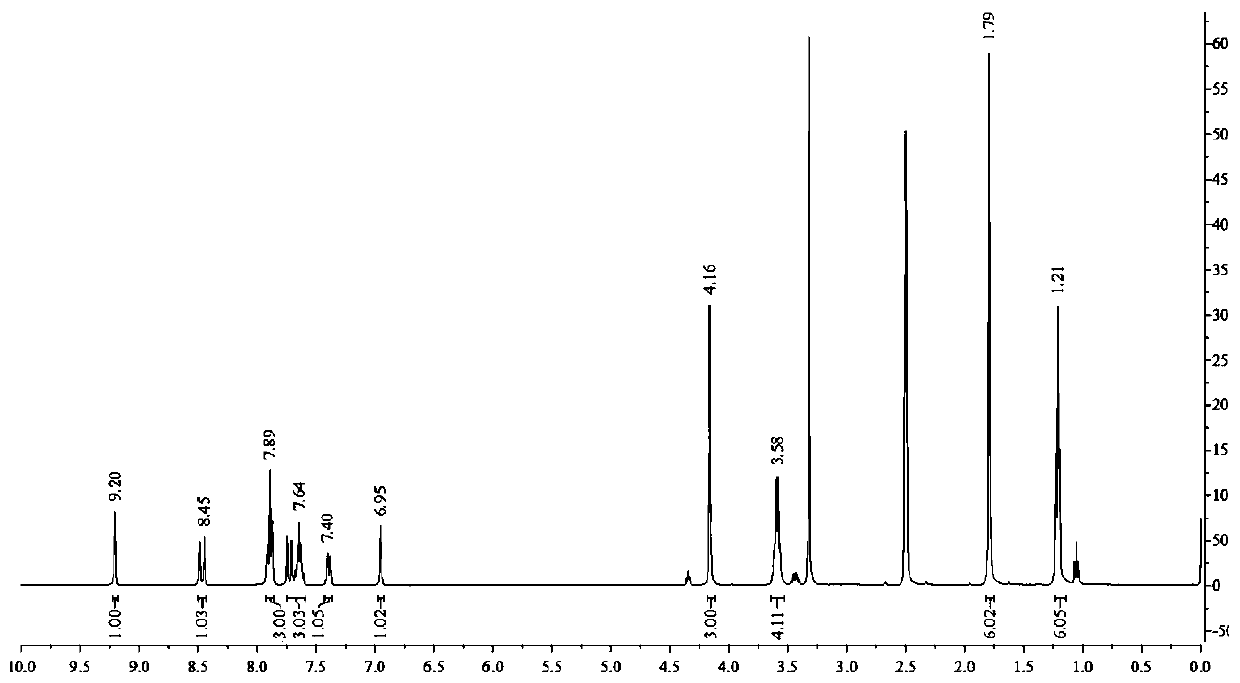
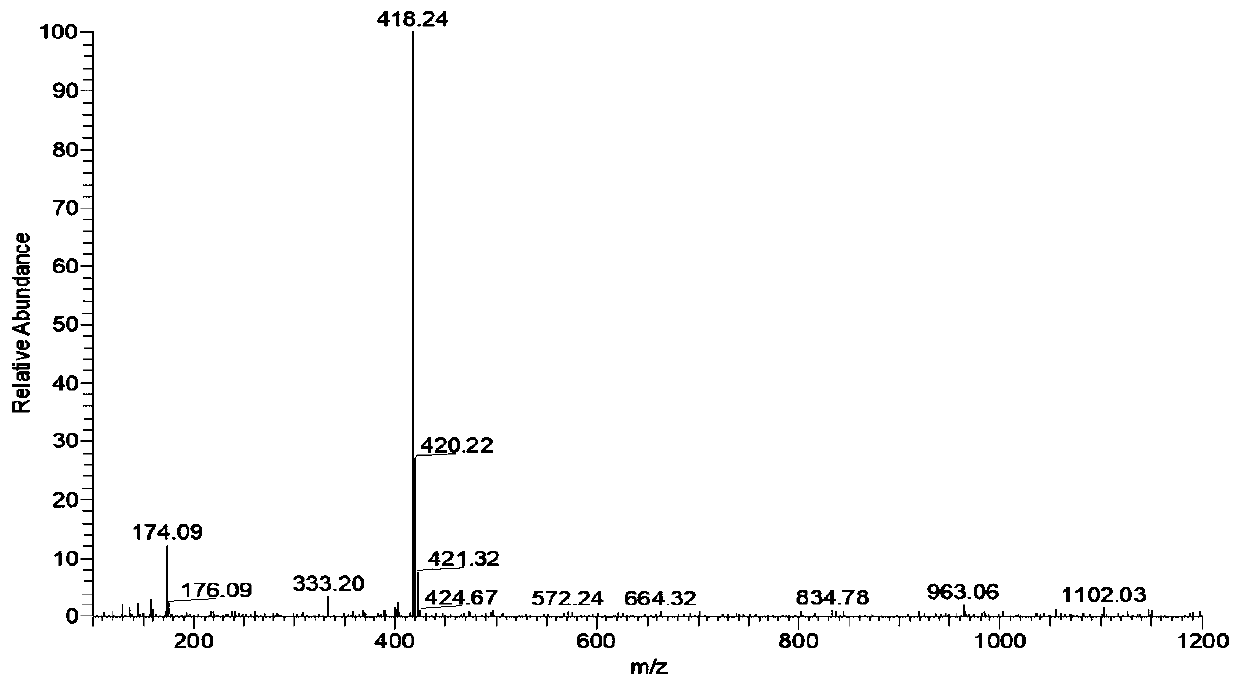
[0046]Add 0.33g (1.2mmol) 7-N,N-diethylamino-2-chloro-3-quinoline formaldehyde, 0.42g (1.4mmol) 1,2,3,3-tetramethyl- 3H-iodine indole salt, add 5mL of n-butanol dropwise, stir to dissolve the solid, add 5 drops of pyridine, stir and reflux for 3h, TLC traces that the reaction is complete, stop stirring, cool to room temperature, filter with suction, and wash the filter cake with cold ethanol ,dry. Silica gel column chromatography, eluting with petroleum ether and ethyl acetate (100 / 3-100, v / v), gave 0.42 g of purple-black solid QIE, with a yield of 83.73%, m.p.: 220.8-222.3°C. IR(KBr), ν / cm -1 : 1588, 1515. 1 H NMR (400MHz. DMSO, δppm), 9.20(s, 1H), 8.49(d, J=16.1Hz, 1H), 7.91~7.87(3H), 7.75~7.61(3H), 7.40(dd, J=9.3 ,2.2Hz,1H), 6.95(d,J=1.8Hz,1H), 4.16(s,3H), 3.58(q,J=6.8Hz,4H), 1.79(s,6H), 1.21(t,, J=7.0Hz, 6H). ESI-MS: 418.24(M).
Embodiment 2
[0048] Preparation of (2-(2-chloro-7-diethylaminoquinolin-3-yl)vinyl-N,3,3-trimethyl-3H-indole (QIE) (microwave reaction method).
[0049] Add 0.33g (1.2mmol) 7-N,N-diethyl-2-chloro-3-quinoline formaldehyde to the test tube, add 0.44g (1.4mmol) 1,2,3,4-tetramethylindole , adding 5 drops of pyridine, reacting in microwave for 20 minutes, cooling, adding ethanol, stirring, filtering to obtain a purple-black solid. Ethanol was added to the solid, stirred at room temperature for 10 hours, suction filtered, and the operation was repeated three times to obtain 0.46 g of a purple-black pure product with a yield of 91.71%. The characterization data are as in Example 1.
Embodiment 3
[0051] (1) Preparation of test solution:
[0052] In a 2mL sample bottle, add 1.0mL double distilled water, then add 1×10 -2 mol / L currently configured cyanide ion (CN - ) standard solution (20μL, 10eq), then add 1.0mL methanol, mix well; finally add 20μL QIE DMF solution (1×10 -3 mol / L), and mix again. After standing for 10 minutes, measure the ultraviolet absorption spectrum and fluorescence emission spectrum (416nm is the excitation wavelength). The above operation, without adding anion solution, is the preparation of blank test solution. Measure the UV absorption spectrum and fluorescence emission spectrum.
[0053] (2) Ultraviolet spectrum and fluorescence spectrum test:
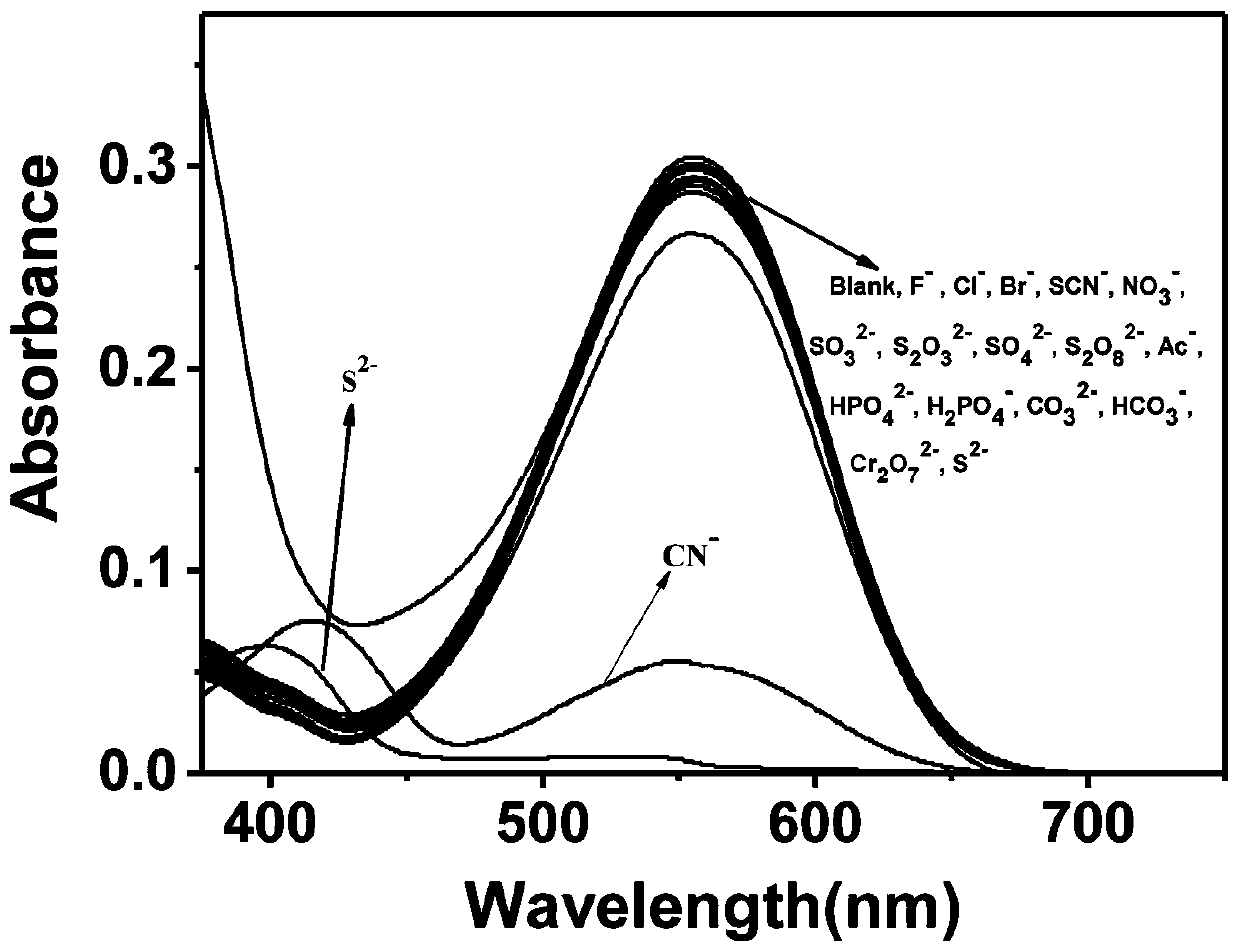
[0054] The blank test solution of QIE has a strong maximum absorption peak at 555nm; when there is cyanide ion, the absorption intensity at 555nm is obviously weakened, see image 3 . The blank test solution of probe QIE has no fluorescence emission peak at 496nm, after adding cyanide ion, the fl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com