Fluorescent probe molecule ML-FP and preparation method and applications thereof
A fluorescent probe and molecular technology, applied in the field of fluorescent probes, can solve the problems of low toxicity and large Stokes shift, and achieve the effects of low cytotoxicity, large Stokes shift and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation and Characterization of Green Fluorescent Protein Fluorescent Probe Molecule ML-FP
[0021] (1) Preparation method of green fluorescent protein fluorescent probe molecule ML-FP
[0022] In an ice-water bath, 18 μL of triethylamine was slowly added dropwise to an anhydrous solution dissolved with morpholine indole fluorescent protein derivative M (47 mg, 0.084 mmol) and 2,4-dinitrobenzenesulfonyl chloride (27 mg, 0.101 mmol). CH 2 Cl 2 After the dropwise addition, the solution was gradually raised to room temperature, stirred and reacted for 12 hours, and the crude product was purified by column chromatography to obtain the fluorescent probe molecule ML-FP with a yield of 63%.
[0023] The synthetic route of compound ML-FP is as follows:
[0024]
[0025] Wherein the morpholine indole fluorescent protein derivative M was prepared according to literature method (Sarah Diab, AhmadM.Abdelaziz, Peng Li, Theodosia Teo, Sunita K.C.Basnet, Ben Noll, ...
Embodiment 2
[0032] Example 2 Application of green fluorescent protein fluorescent probe molecule ML-FP
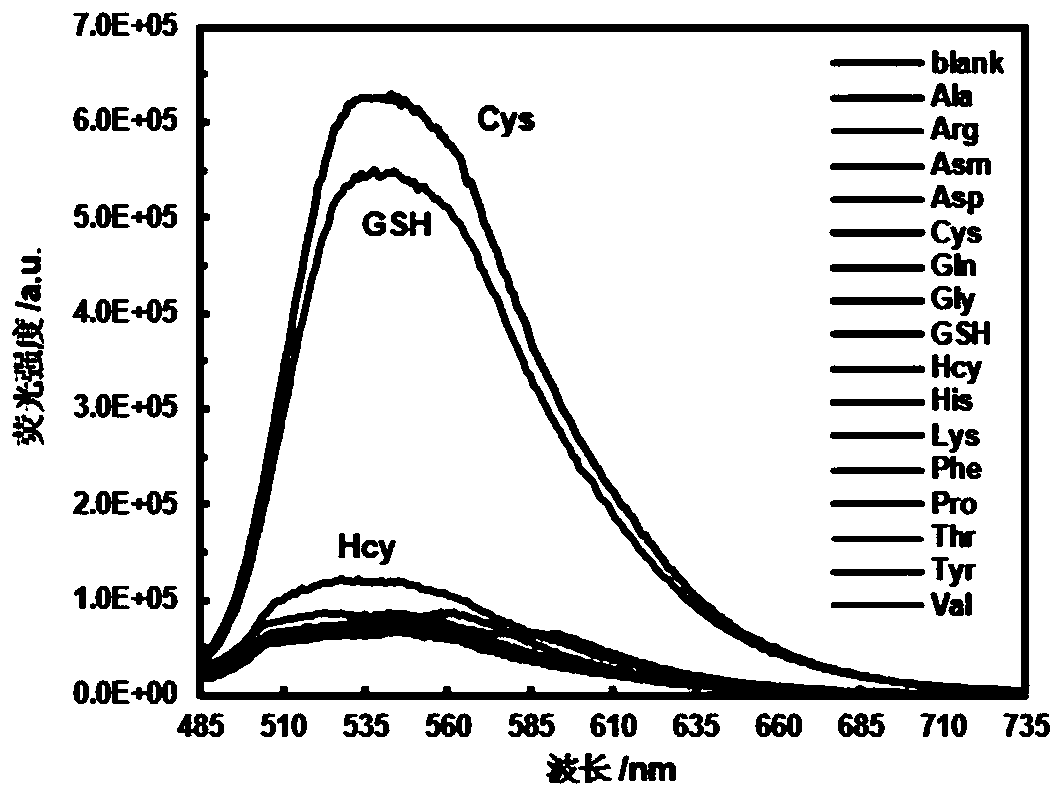
[0033]The maximum absorption wavelength of the probe molecule ML-FP is 470nm, the maximum emission wavelength is 542nm, and the Stokes shift is 72nm. The large Stokes shift makes the probe ML-GFP have excellent properties such as anti-background interference and high sensitivity. . At 25°C, incubate the probe ML-GFP with 5 times the equivalent of various biothiols and amino acids for more than 30 minutes, such as L-alanine (Ala), L-arginine (Arg), L-day Partine (Asm), L-Aspartic Acid (Asp), Cysteine (Cys), L-Glutamine (Gln), Glycine (Gly), Glutathione (GSH), Homocysteine Acid (Hcy), L-Histidine (His), L-Lysine (Lys), L-Phenylalanine (Phe), Proline (Pro), L-Threonine (Thr), Tyrosine amino acid (Tyr) and valine (Val). figure 1 In the buffer solution of the fluorescent probe molecule ML-GFP (the pH of the buffer solution is 7.2, the solvent is a mixed solvent composed of water and et...
Embodiment 3
[0034] Example 3 Application of lysosome targeting active thiol fluorescent probe of green fluorescent protein fluorescent probe molecule ML-FP
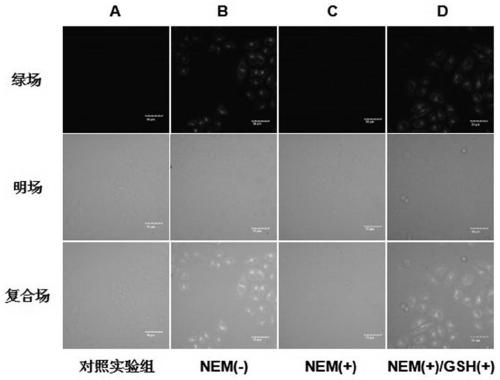
[0035] The probe molecule ML-FP has good cell permeability, can be quickly located in lysosomes, and can quantitatively monitor biothiols in lysosomes. figure 2 Lysosome co-localization of probe molecule ML-FP and commercial red lysosome localization reagent Lyso-Tracker Red (Nanjing Lattice Chemical Reagent Company) in Bel-7402 cells (purchased from Nanjing Lattice Chemical Reagent Company) Imaging photos. The red lysosome localization reagent Lyso-Tracker Red and ML-FP were simultaneously injected into Bel-7402 living cells for co-staining, and the cells were incubated in an incubator for 30 minutes. After washing the cells three times with PBS buffer solution, the fluorescent signals under the green field and red field were observed by confocal microscope. Such as figure 2 As shown, cells with green and red fluorescent signal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com