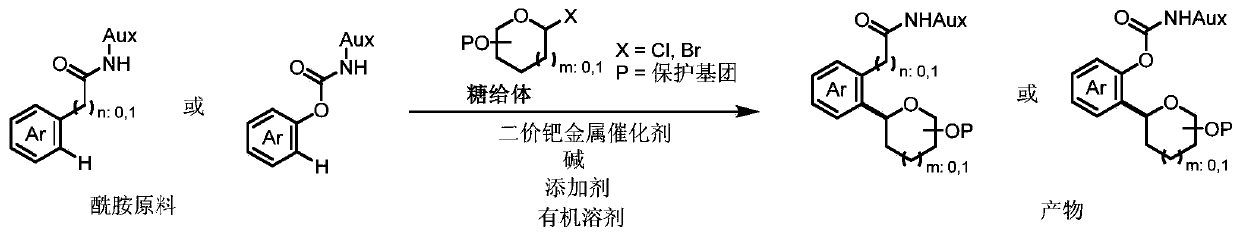
Synthesis method of aryl carbon glycoside compound
A technology of aryl carbon glycosides and synthesis methods, which is applied in the fields of metal catalysis and medicinal chemistry applications, can solve the problems of unfavorable environment-friendly development, poor atom economy, inconvenient use, etc., achieve stable and easy-to-obtain acceptor structures, and promote carbon The effect of hydrogen activation reaction and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0045]
[0046] Add amide material 1-s (26.2 mg, 0.1 mmol, 1.0 equiv), chlorosugar (Donor-1) (112 mg, 0.2 mmol, 2.0 equiv), palladium acetate (2.2 mg, 0.01 mmol, 0.1equiv), acetyl isoleucine (Ac-Ile-OH) (5.2mg, 0.03mmol, 0.3 equiv), potassium acetate (14.7mg, 0.15mmol, 1.5equiv), add 1ml of solvent, cover the reaction vial (There is no need for particularly strict anhydrous and oxygen-free conditions), and stirred at 60°C for 6 hours. After the reaction was complete, the reaction bottle was cooled to room temperature, filtered, concentrated, and separated by column chromatography to obtain the product as a colorless oil. The preparation steps of Examples 1-9 are basically the same, the difference lies in the use of different reaction solvents.
[0047] The investigation of table 1 reaction solvent
[0048]
[0049] a:NMR Yield
[0050] It can be found from Examples 1-9 that when the reaction solvent is PhMe, the yield of Example 6 is the best.
Embodiment 10-17
[0052] The preparation method of embodiment 10-17 is basically the same as the preparation method of embodiment 6, and the only difference is that the divalent palladium metal catalyst selected in embodiment 10-17 is different, specifically as shown in table 2
[0053] The screening of table 2 divalent palladium metal catalyst
[0054]
[0055] a: NMR Yield
[0056] Can find by embodiment 10-17, when divalent palladium metal catalyst is Pd(OAc) 2 , the productive rate of embodiment 6 is the best.
Embodiment 18-23
[0058] The preparation method of Examples 18-23 is basically the same as the preparation method of Example 6, the only difference is that the bases used in Examples 18-23 are different, as shown in Table 3.
[0059] The investigation of table 3 alkali
[0060]
[0061] a: NMR Yield
[0062] Can find by embodiment 18-23, when alkali is potassium acetate, the productive rate of embodiment 6 is the best.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com