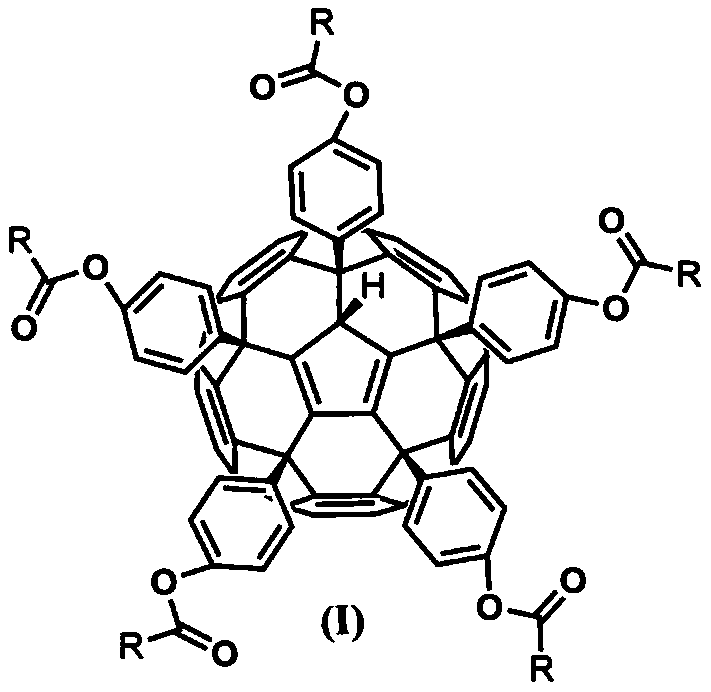
Fullerene phenolic ester derivative as well as preparation method and application thereof
A technology of fullerene phenol esters and derivatives, which is applied in the field of solid rocket propellants, fullerene phenol ester derivatives and their preparation, can solve the problems of poor stability and adaptability, and achieve stable performance and preparation The method is simple and convenient, and the effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
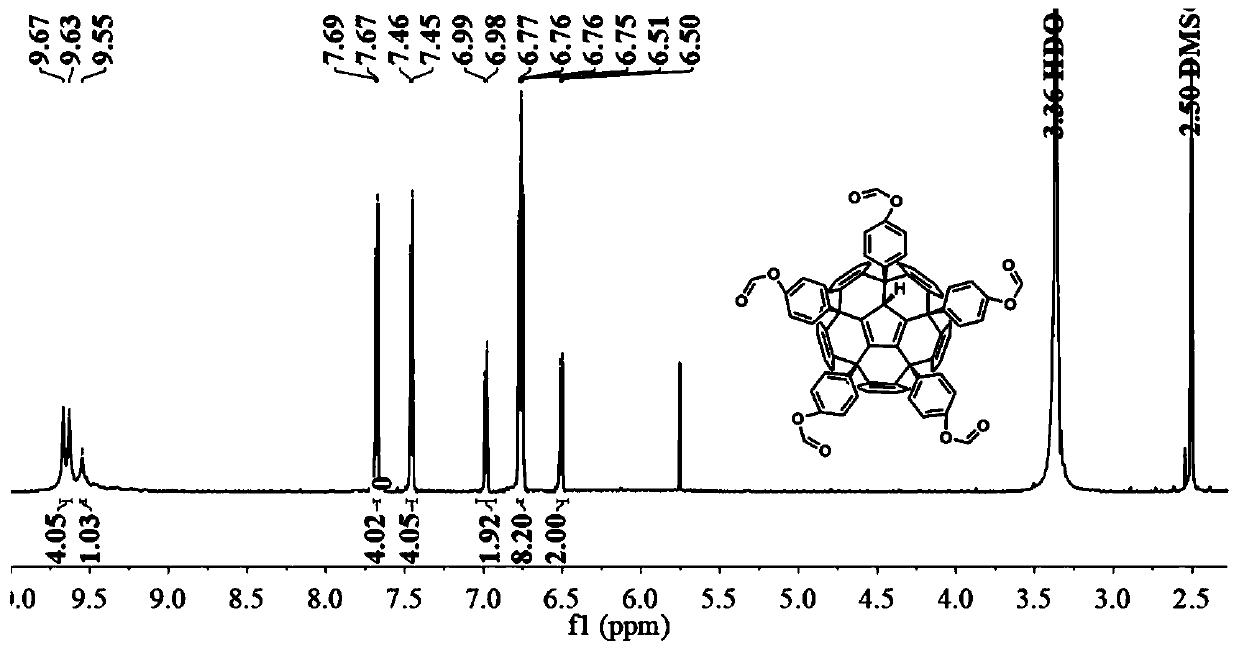
[0025] The preparation method of formic acid phenyl fullerene (a):
[0026] At a room temperature of 25° C., 0.05 mmol of hexachlorofullerene was dissolved in 20 mL of nitrobenzene, and the mixed solution was stirred and dissolved under nitrogen protection for 2 hours. After being completely dissolved, 15 mmol of phenyl formate and 4.55 mmol of titanium tetrachloride were added to the mixed solution, and the temperature of the oil bath was raised to 100°C. The reaction was stirred under nitrogen protection until thin layer chromatography showed that the reactant was completely consumed, and the reaction solution changed from orange to dark brown. After the reaction, excess solvent was removed by distillation under reduced pressure to obtain a reddish-brown crude product. The crude product was dissolved in a small amount of carbon disulfide, and the orange-red product was obtained by separation by silica gel column chromatography, and the eluent was ethyl acetate / carbon disulf...
Embodiment 2
[0028] The preparation method of formic acid phenyl fullerene (a):
[0029] At a room temperature of 25° C., 0.05 mmol of hexachlorofullerene was dissolved in 20 mL of nitrobenzene, and the mixed solution was stirred and dissolved under nitrogen protection for 2 hours. After completely dissolving, 15 mmol of phenyl formate and 6 mmol of anhydrous aluminum trichloride were added to the mixed solution, and the temperature of the oil bath was raised to 100°C. The reaction was stirred under nitrogen protection until thin layer chromatography showed that the reactant was completely consumed, and the reaction solution changed from orange to dark brown. After the reaction, excess solvent was removed by distillation under reduced pressure to obtain a reddish-brown crude product. The crude product was dissolved in a small amount of carbon disulfide, and the orange-red product was obtained by separation by silica gel column chromatography, and the eluent was ethyl acetate / carbon disulf...
Embodiment 3
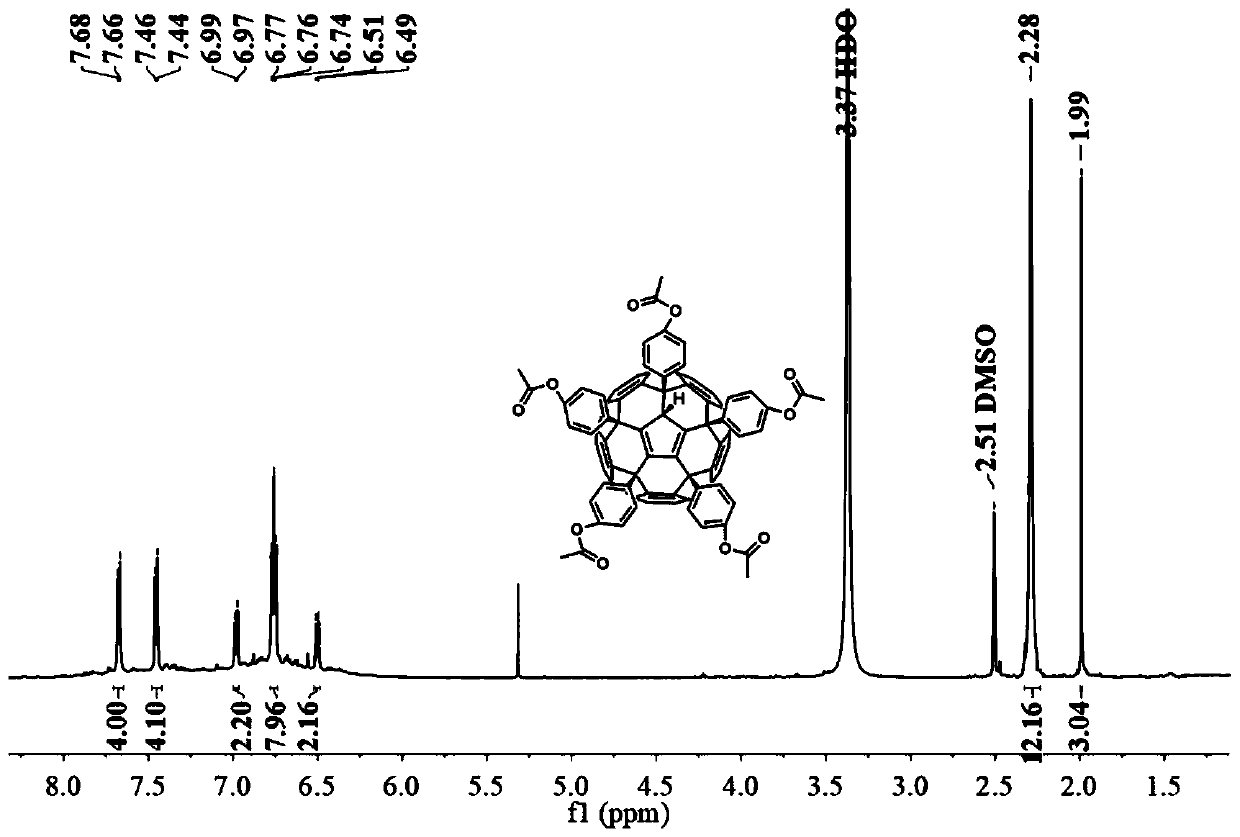
[0031] The preparation method of phenyl acetate fullerene (b):
[0032] At a room temperature of 25° C., 0.05 mmol of hexachlorofullerene was dissolved in 20 mL of nitrobenzene, and the mixed solution was stirred and dissolved under nitrogen protection for 2 hours. After being completely dissolved, 15 mmol of phenyl acetate and 4.55 mmol of titanium tetrachloride were added to the mixed solution, and the temperature of the oil bath was raised to 100°C. The reaction was stirred under nitrogen protection until thin layer chromatography showed that the reactant was completely consumed, and the reaction solution changed from orange to dark brown. After the reaction, excess solvent was removed by distillation under reduced pressure to obtain a reddish-brown crude product. The crude product was dissolved in a small amount of carbon disulfide, and the orange-red product was obtained by separation by silica gel column chromatography, and the eluent was ethyl acetate / carbon disulfide ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com