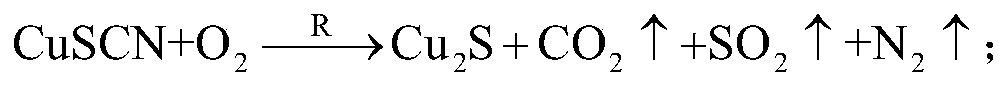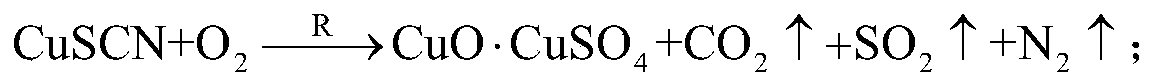Method for catalyzing and oxidizing thiocyanide
A thiocyanide and cyanide technology, applied in the chemical industry, can solve the problems of high ozone cost, high equipment material requirements, large energy consumption, etc., and achieve the effects of no secondary pollution, less equipment investment and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
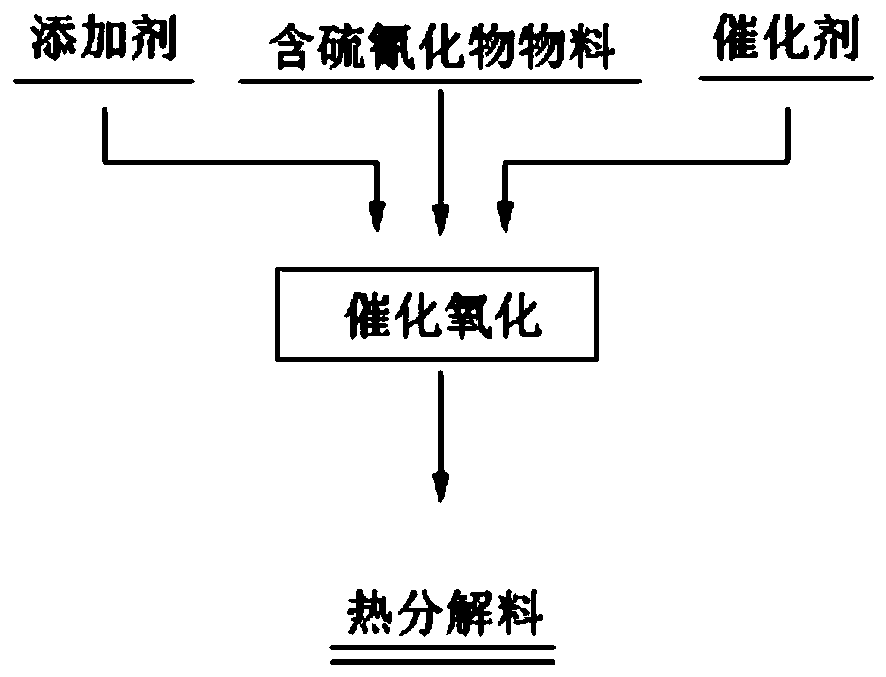
[0047] A method for catalytic oxidation of thiocyanide, comprising the steps of:
[0048] (1) With the solid reagent waste material containing 98.5% CuSCN as the raw material, add catalyst iron oxide and additive lime in the raw material, the catalyst is specifically copper oxide, the iron oxide and the thiocyanide contained in the raw material The mass ratio is 2:1, the mass ratio of available calcium oxide in the lime to the raw material is 4:10; mix evenly to make mixed raw material;
[0049] (2) Put the mixed raw material into a rotary kiln with an air atmosphere, and heat it from room temperature to 300°C at a heating rate of 6°C / min for thermal decomposition. When the temperature reaches 300°C, keep it warm for 120min to remove thiocyanide. Obtain pyrolysis material and pyrolysis gas; chemical reactions that occur during pyrolysis include:
[0050]
[0051]
[0052]
[0053] CaO+SO 2 →CaSO 3 ;
[0054] (3) directly stockpiling the obtained thermal decomposit...
Embodiment 2
[0057] A method for catalytic oxidation of thiocyanide, comprising the steps of:
[0058] (1) With the solid reagent waste material that contains 98.5% KSCN as raw material, add cobalt oxide and additive lime in raw material, the rhodanide contained in described cobalt oxide and raw material is counted as 1: 1 by mass ratio, in described lime The mass ratio of effective calcium oxide to raw materials is 6:10; mix evenly to make mixed raw materials;
[0059] (2) Put the mixed raw material into a rotary kiln with an air atmosphere, heat it to 370°C at a heating rate of 15°C / min for thermal decomposition, and when the temperature reaches 370°C, keep it warm for 15 minutes to remove thiocyanide and obtain heat Decomposition materials; chemical reactions that occur during pyrolysis include:
[0060]
[0061] CaO+SO 2 →CaSO 3
[0062] (3) directly stockpiling the obtained thermal decomposition material.
[0063] After testing, it was found that in the thermal decomposition p...
Embodiment 3
[0065] A method for catalytic oxidation of thiocyanide, comprising the steps of:
[0066] (1) With the solid reagent waste material that contains 98.5% KSCN as raw material, add nickel oxide and additive lime in raw material, the sulfur cyanide contained in described nickel oxide and raw material is counted as 1: 2 by mass ratio, in described lime The mass ratio of effective calcium oxide to raw materials is 8:10; mix evenly to make mixed raw materials;
[0067] (2) Put the mixed raw material into a roasting furnace with an oxygen atmosphere, heat it to 450°C at a heating rate of 20°C / min for thermal decomposition, and when the temperature reaches 450°C, remove the thiocyanide to obtain the thermally decomposed material; The chemical reactions that occur include:
[0068]
[0069] CaO+SO 2 →CaSO 3
[0070] (3) directly stockpiling the obtained thermal decomposition material.
[0071] After testing, it was found that in the thermal decomposition process, the content of ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap