Quinoline triarylamine and preparation method thereof
A technology of quinolinyl triarylamine and triarylamine, which is applied in the field of catalytic organic synthesis, can solve the problems of low reaction efficiency, difficult synthesis, and difficult oxidation addition of metal catalysts, etc., and achieves the effect of high yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
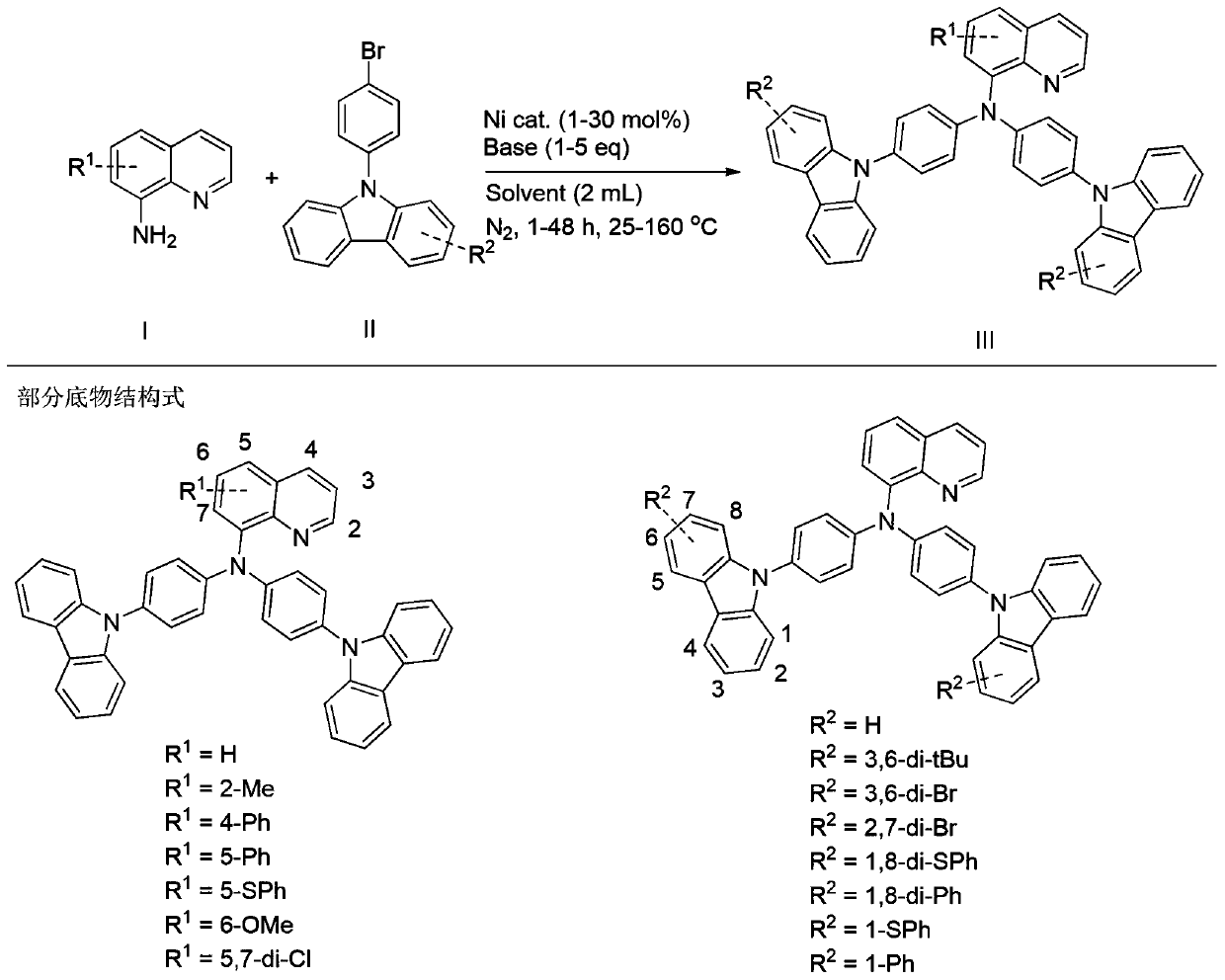
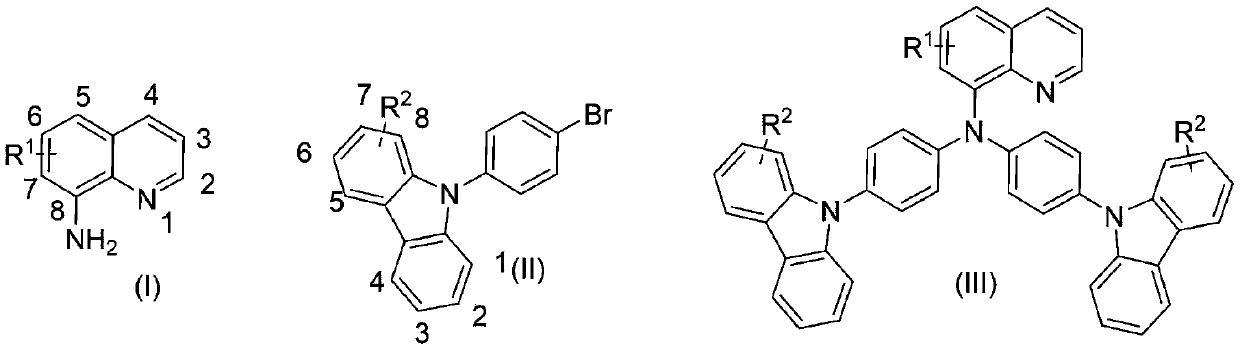
[0024] Add 1 magnetic stir bar, 8-amino-5-thiophenylquinoline (0.3mmol), 9-(4-bromophenyl)carbazole (0.6mmol), and nickel chloride (0.06mmol) to the 10mL reaction tube. ), potassium carbonate (1.2 mmol), vacuum and fill with nitrogen 3 times, add tetrahydrofuran (2 mL) under nitrogen atmosphere, and react at 100°C for 24 hours. After the reaction, it was quenched by adding saturated aqueous ammonium chloride solution, extracted with ethyl acetate, dried, concentrated, and separated by column chromatography to obtain N,N-bis(4-(9H-carbazol-9-yl)phenyl)- The yield of 5-(phenylthio)-8-aminoquinoline was 97%.
preparation example 2
[0026] Add 1 magnetic stir bar, 8-aminoquinoline (0.3mmol), 9-(4-bromophenyl)carbazole (0.6mmol), nickel bromide (0.06mmol), potassium carbonate (1.2mmol) into the 10mL reaction tube. mmol), vacuum and fill with nitrogen 3 times, add tetrahydrofuran (2mL) under nitrogen atmosphere, and react at 100°C for 24 hours. After the reaction, it was quenched by adding saturated aqueous ammonium chloride solution, extracted with ethyl acetate, dried, concentrated, and separated by column chromatography to obtain N,N-bis(4-(9H-carbazol-9-yl)phenyl)- The yield of 8-aminoquinoline was 95%.
preparation example 3
[0028] Add 1 magnetic stir bar, 8-amino-6-methoxyquinoline (0.3mmol), 9-(4-bromophenyl)carbazole (0.6mmol), nickel fluoride (0.03mmol) to the 10mL reaction tube ), potassium carbonate (0.9 mmol), evacuated and filled with nitrogen 3 times, added tetrahydrofuran (2 mL) under nitrogen atmosphere, and reacted at 100°C for 24 hours. After the reaction, it was quenched by adding saturated aqueous ammonium chloride solution, extracted with ethyl acetate, dried, concentrated, and separated by column chromatography to obtain N,N-bis(4-(9H-carbazol-9-yl)phenyl) -6-(Methoxy)-8-aminoquinoline, the yield was 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com