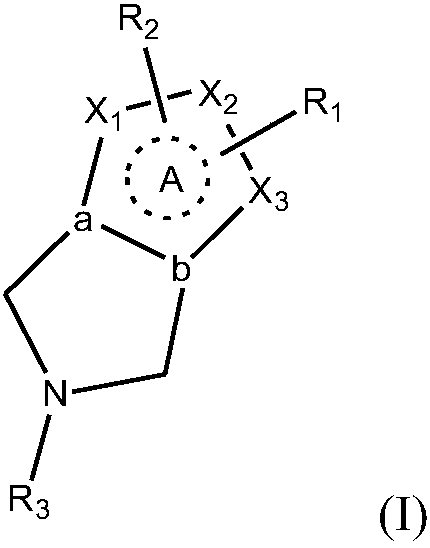
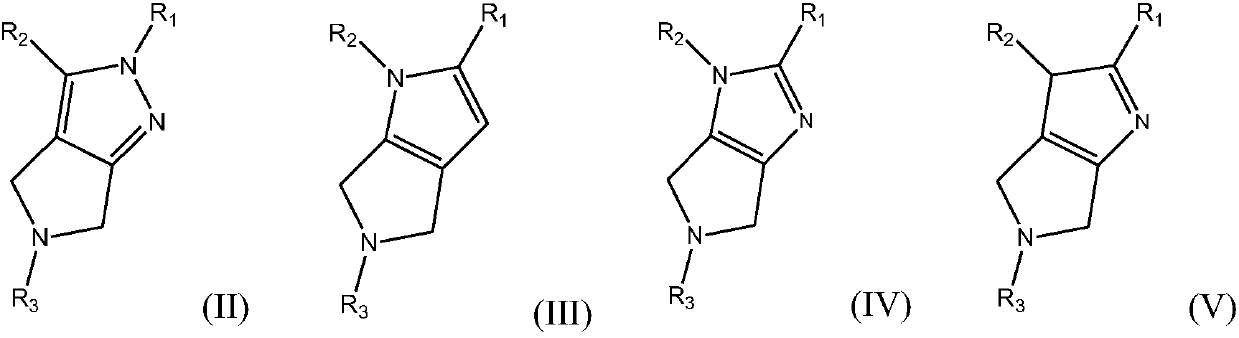
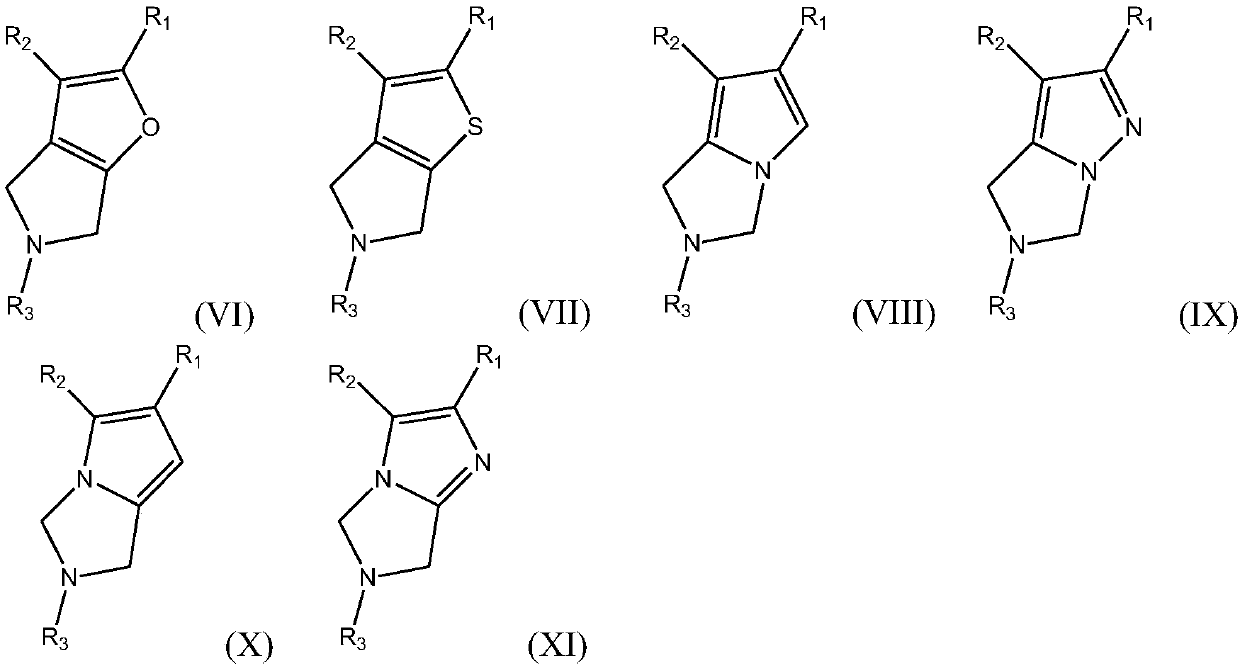
Azacycle-containing compound and preparation method and usage thereof
A compound, cycloalkyl technology, applied in the field of medicine, can solve the problems of repeated disease, prolonged disease cure time, and patient response differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] The preparation of embodiment 1 compound 101
[0135]
[0136] Compound 101a (10g, 65.4mmol), 2-fluoroiodobenzene (29g, 130.7mmol), cuprous iodide (24.8g, 130.7mmol), tripotassium phosphate (27.7g, 130.7mmol) and N,N'- Dimethylethylenediamine (5.8 g, 65.4 mmol) was added into toluene (200 mL), and refluxed under argon atmosphere for 24 h. The reaction solution was cooled and filtered, the filtrate was washed with water (100 mL×2), and the aqueous layer was extracted with ethyl acetate (200 mL×1). The organic phase was washed with saturated brine (200mL×1), dried over anhydrous sodium sulfate, filtered, concentrated, and the residue was subjected to column chromatography (petroleum ether / ethyl acetate=30 / 1 to 20 / 1) to obtain compound 101b (yellow solid). MS m / z(ESI):248.2[M+H].
[0137] Compound 101b (1.7g, 6.88mmol) was added in dichloromethane (30mL), cooled to -78°C, and N-bromosuccinimide (1.22g, 6.88mmol) was slowly added dropwise in dichloromethane (20mL ) s...
Embodiment 2
[0145] The preparation of embodiment 2 compound 114
[0146]
[0147] Compound 114a (476mg, 3.0mmol) and o-fluorobenzoyl chloride (426mg, 3.0mmol) were dissolved in dry toluene (6mL) under argon atmosphere, then trimethyl phosphite (372mg, 3mmol) was added dropwise in dry toluene (9mL) solution, react at room temperature for 2h. The reaction solution was concentrated, and the residue was subjected to column chromatography (petroleum ether / ethyl acetate=3 / 2) to obtain compound 114b (370 mg, yellow solid). MS m / z(ESI):375.1[M+1].
[0148] Compound 114b (800mg, 2.14mmol) and benzylhydrazine dihydrochloride (625mg, 3.2mmol, 1.5eq.) were suspended in toluene (3mL), refluxed for 2h, the solvent was evaporated under reduced pressure, and the residue was subjected to column chromatography (Petroleum ether / ethyl acetate=5 / 1) to obtain compound 114c (633 mg, yellow oil). MS m / z(ESI):369.2[M+1].
[0149] Compound 114c (920 mg, 2.5 mmol) was dissolved in 10 mL of acetonitrile, 40 mL ...
Embodiment 3
[0154] The preparation of embodiment 3 compound 104d
[0155]
[0156] Compound 104a (500g, 2.70mol) was dissolved in N,N-dimethylformamide dimethyl acetal (3.5L), refluxed for 3h, the reaction solution was evaporated to remove the solvent under reduced pressure, and the residue was added with n-hexane (500mL) and stirred , filtered, washed with cyclohexane (3 L), and dried in vacuo at 40° C. to give compound 104b (yellow solid). MS m / z(ESI): 241.3[M+1]. 1 H NMR (400MHz, CDCl 3 )δ7.31(s,1H),4.55(d,J=17.3Hz,2H),3.83(d,J=20.4Hz,2H),3.07(d,J=23.4Hz,6H),1.49(d, J=6.1Hz,9H)
[0157] Compound 104b (335g, 1.40mmol) was dissolved in toluene (1.675L), and hydrazine hydrate (81mL, 1.67mol) was added dropwise at 45°C, kept at 45°C for overnight reaction, a large amount of solid precipitated, filtered, and washed with cyclohexane (1.5L) , dried in vacuo at 50°C to give compound 104c (yellow solid). 1 H NMR (400MHz, CDCl 3 )δ7.24(s,1H),6.66(s,1H),6.16(s,1H),3.62(d,J=11.0Hz,2H),3.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com