Preparation method of stable amino acid-ferrous complex
A technology of amino acid ferrous and complexes, applied in the preparation of sulfide, organic chemistry, etc., can solve the problem of removing impurity ions and dissolved oxygen in water, stability control of amino acid ferrous products, and increasing ferrous Difficulty and workload of anti-oxidation, to reduce the risk of ferrous oxidation, ensure high ferrous quality, and ensure the effect of anti-oxidation function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of ferrous methionine complex: Add 602.02kg of industrial desalted water (conductivity 2.8us / cm) into a 2000L reactor, then raise the temperature to 95°C, keep it warm for 30~60min, and then inject nitrogen gas for 5~15min at the same time, analyze The residual amount of oxygen in the water was not detected. Then add 301.01kg of DL-methionine crystals (2000 moles) with a content of 99wt% and 310.02kg (2000 moles) of anhydrous ferrous sulfate with a content of 98wt%, stir and dissolve completely, add reduced iron powder 0.448kg (8 moles), and Keep warm at 40~95°C for 20~60min, add 3.41kg (20mol) of additive 2-hydroxy-4-methylthiobutyric acid, adjust the pH of the reaction solution to 2~5, digest the residual reduced iron powder, and stir for 10~30min , to obtain DL-methionine complex ferrous salt aqueous solution, the concentration of DL-methionine complex ferrous salt in the aqueous solution is 49.7wt%.
[0038] Spray drying treatment of ferrous methionine ...
Embodiment 2
[0040] Preparation of ferrous glycinate complex: add 451.52kg of industrial desalted water (conductivity 3.2us / cm) into a 2000L reactor, then raise the temperature to 95°C, keep it warm for 30~60min, and pass nitrogen gas for 5~15min at the same time in the later stage, and analyze the water Residual amount of oxygen, not detected. Then add 151.66kg of glycine crystals (2000 moles) with a content of 99wt% and 573.22kg (2000 moles) of ferrous sulfate heptahydrate with a content of 97wt%, stir and dissolve completely, add reduced iron powder 0.448kg (8 moles), at 40~ Insulate at 95°C for 20-60 minutes, add 2.56 kg (15 moles) of auxiliary agent 2-hydroxy-4-methylthiobutyric acid, adjust the pH of the reaction solution to 2-5, digest the residual reduced iron powder, and stir for 10-30 minutes to obtain The aqueous solution of the ferrous glycinate complex, the concentration of the ferrous glycinate complex in the aqueous solution is 45.53wt%.
[0041] Spray drying treatment of f...
Embodiment 5
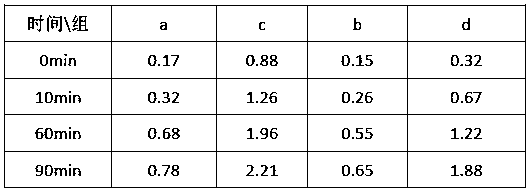
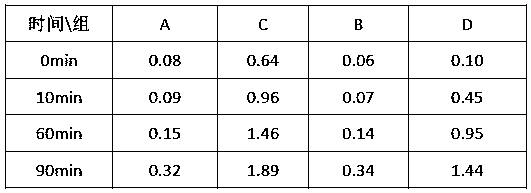
[0047] Stability Test 1:
[0048] When ferrous ions exist as stable complexes and chelates, they are not easily oxidized whether in solid state or in aqueous solution, but the free ferrous ions are easily oxidized to ferric iron by air or oxygen in solution. , ferrous iron is oxidized and consumed, which further promotes the analysis to go forward, resulting in the consumption of ferrous iron complexes and the conversion of ferrous iron to unusable ferric iron. The experiment mainly tracks the analysis reaction of the product to judge its stability by measuring the content of ferric iron in the solution.
[0049] Take 20g of the ferrous complexes obtained in Examples 1, 2, 3, and 4 and transfer them into 100ml beakers, add 20ml of normal temperature deoxidized deionized water, stir and dissolve them, record them as groups A, B, C, and D, and place them on the operating table , respectively at 10min, 60min, and 90min, sample and analyze the content of ferric iron in each group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com