Method for preparing MGO-laccase
A technology of laccase and graphene, applied in biochemical equipment and methods, chemical instruments and methods, enzymes, etc., can solve problems affecting the accessibility of enzymes and mediators, and achieve industrial production, separable, high The effect of the bleaching effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 Preparation of Magnetic Graphene Oxide Immobilized Laccase
[0035] 1 Extraction of CotA crude enzyme solution
[0036] Expression of CotA laccase protein. A strain of Bacillus subtilis cjp3 with laccase activity was isolated from the black liquor of a paper mill, and the CotA laccase gene was cloned from the cjp3 strain and induced to express in E. mM CuSO4, 200rpm, induced for 10h at 25°C, and ultrasonically crushed to obtain a crude enzyme solution. The medium is LB+Kan liquid medium.
[0037] LB+Kan liquid medium: tryptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L, pH=7±0.2, sterilized at 121°C under high temperature and high pressure for 20 minutes. After cooling, filter-sterilized Kanamycin Sulfate (Kan) was added to a final concentration of 100 μΜ.
[0038] 2 Preparation of Graphene Oxide
[0039] GO is usually prepared according to a modified Hummers method. The preparation of specific graphene oxide is divided into three stages, namely...
Embodiment 2
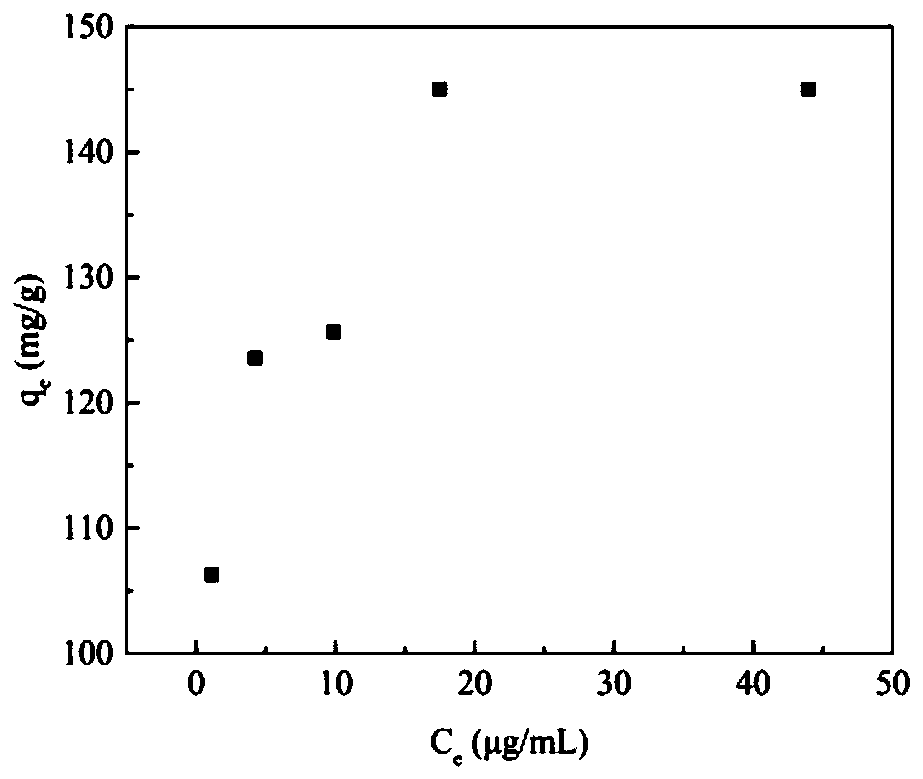
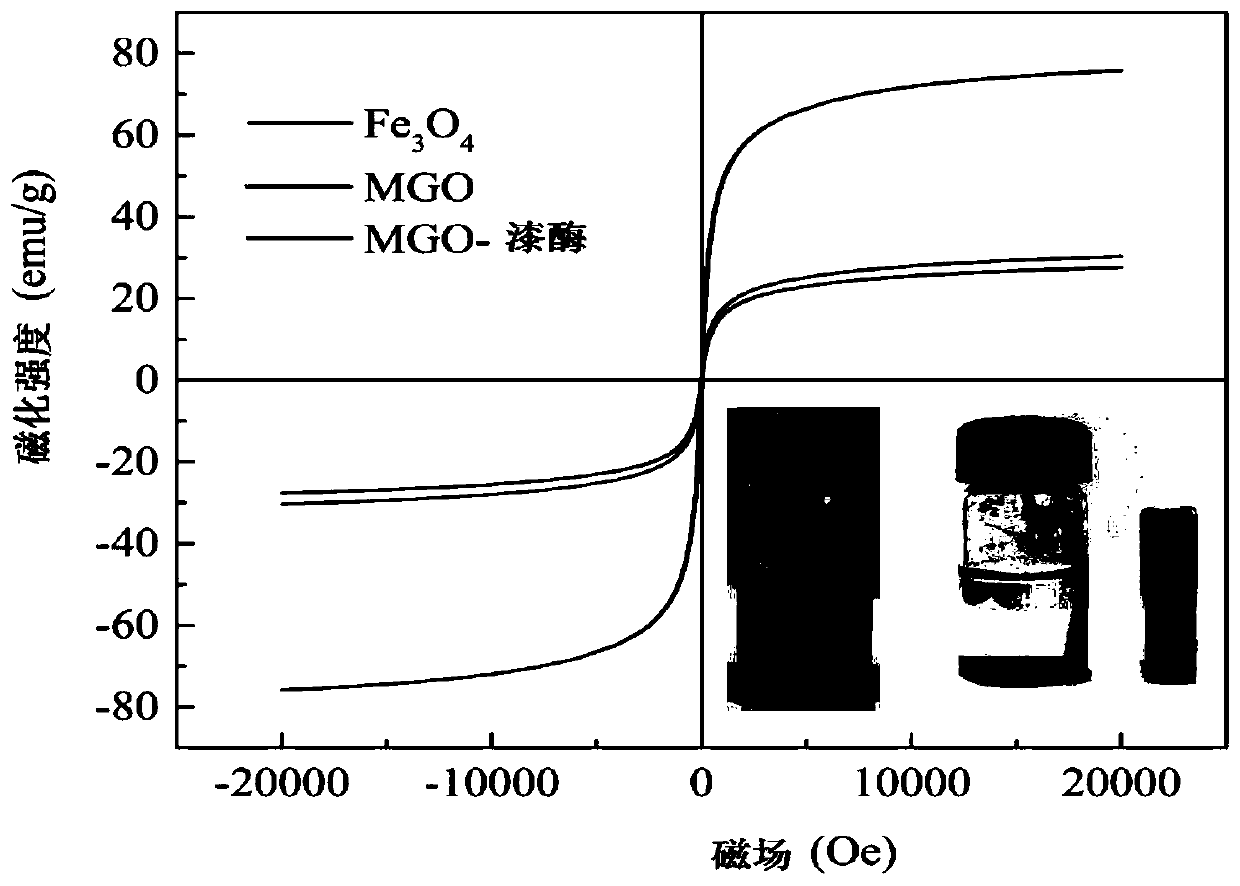
[0063] The characterization of embodiment 2 MGO-laccase
[0064] The samples of ferric oxide, graphene oxide, MGO and MGO-laccase were ultrasonically obtained, and the low-concentration and uniform dispersion liquid was added dropwise to the copper grid, and the samples were photographed under the electron microscope after drying. The morphology and structural characteristics of the sample on the porous carbon-coated copper grid were further observed by using a microscope system operated at 120 kV with a transmission electron microscope JEM-1400 produced by Japan JEOL Company.
[0065] Such as Figure 4 As shown in (a), the TEM image clearly shows that graphene oxide is stacked together in a single layer or several layers, and the surface of the graphene oxide sheet is very smooth, as thin as a gauze. Depend on Figure 4 (b) It can be seen that Fe 3 o 4 The particles are spherical, and its average particle diameter is about 20nm. while GO and Fe 3 o 4 The composite imag...
Embodiment 3
[0066] Embodiment 3 MGO-laccase degradation dye
[0067] 1 Conditions for the decolorization of malachite green by MGO-laccase
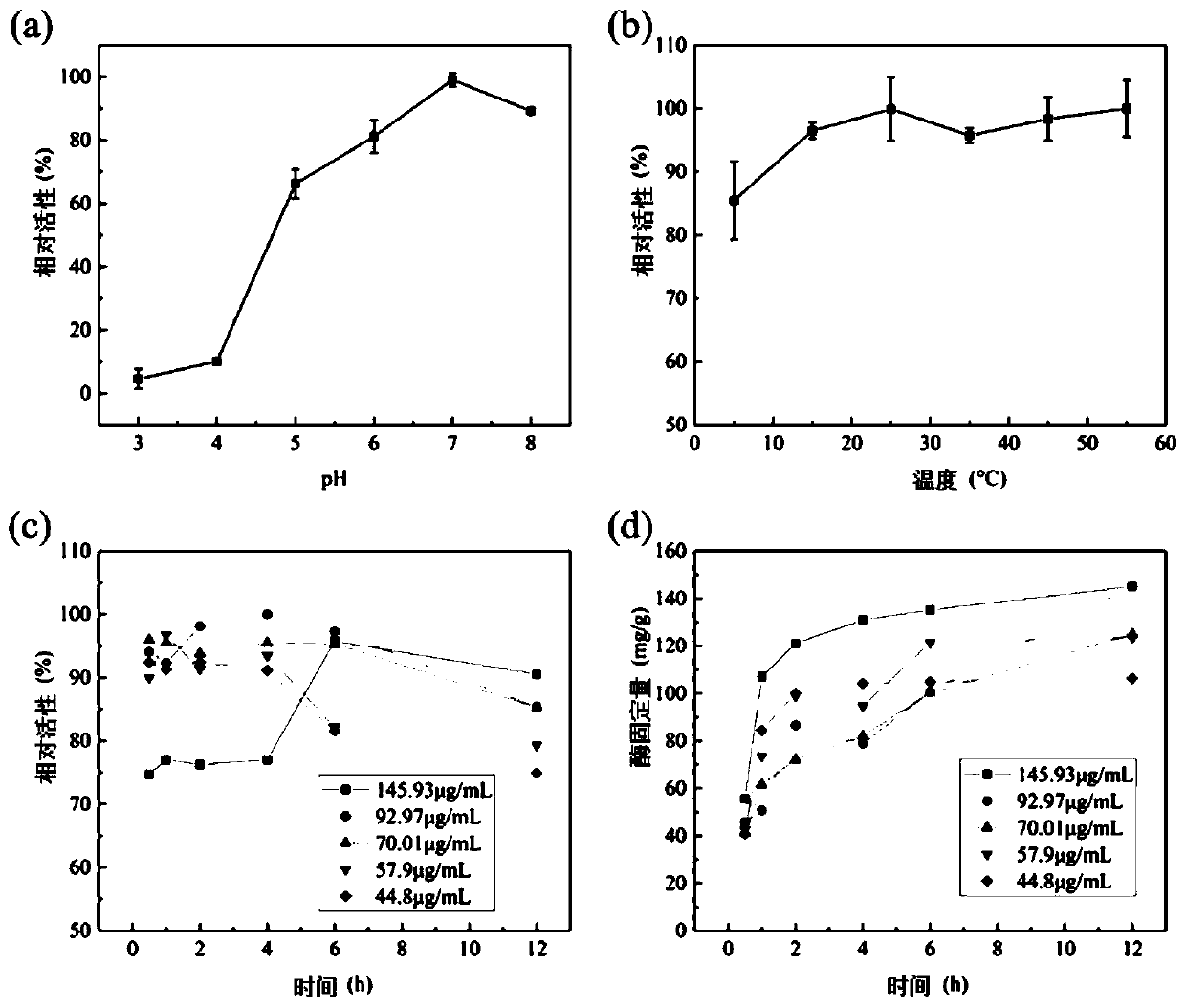
[0068] The ability of MGO-laccase to decolorize MG dye was measured using a TU1810 UV-Vis spectrophotometer. The decrease in absorbance due to MG loss was measured at a wavelength of maximum absorption of 617 nm. Suspend 0.9 mg / mL free laccase, immobilized laccase (about 10 mg) or heat-inactivated immobilized enzyme in 10 mL of 50 mg / L dye solution in the presence or absence of ABTS. The reaction mixture was incubated with constant shaking (150 rpm). The absorbance of the dye is measured at regular time intervals.
[0069] When the temperature was 30°C, about 10 mg of immobilized laccase was incubated in malachite green dye (50 mg / L) in a series of different pH buffers for 1 h. Such as Figure 5 As shown in (a), it shows that the decolorization rate of the immobilized laccase is the best at pH 6, as high as 83.3%. After determining the most sui...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com