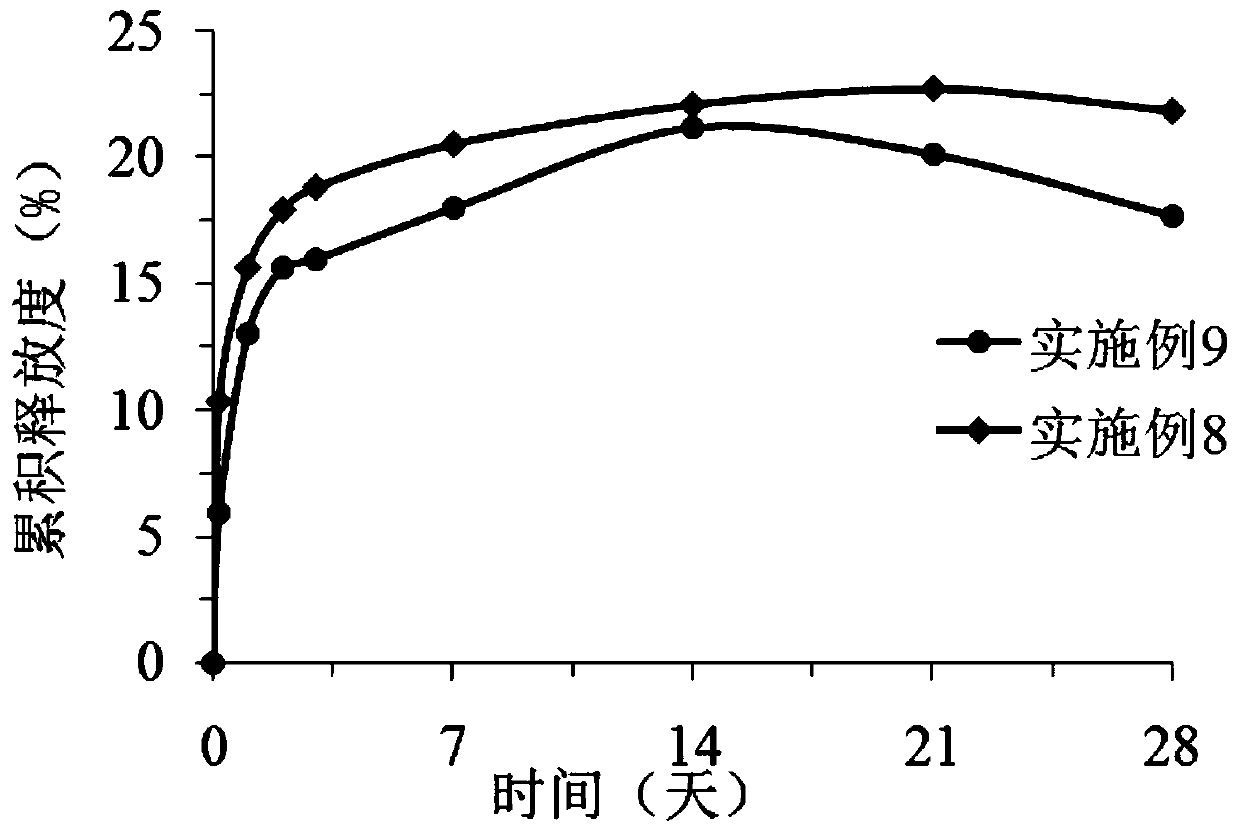
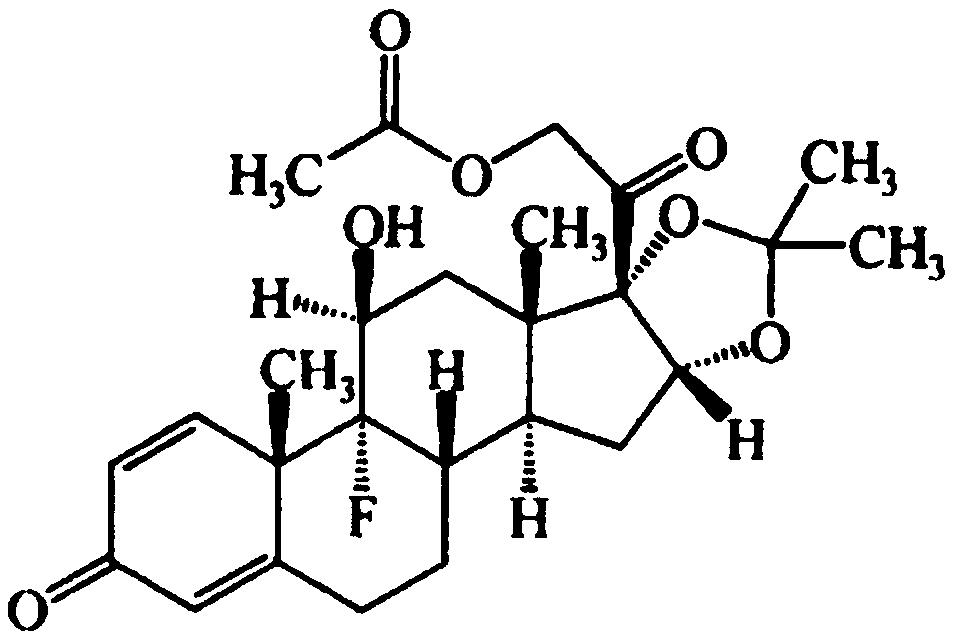
Triamcinolone acetonide acetate sustained release microsphere and preparation method thereof
A technology of triamcinolone acetonide acetate and sustained-release microspheres, which is applied in pharmaceutical formulations, microcapsules, medical preparations with inactive ingredients, etc., can solve the problems of poor patient compliance, short preparation release time, etc., and achieve slow drug loading. Release, reduce drug loss, good form effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 70.8 mg of triamcinolone acetonide acetate and 187.5 mg of PLA dissolved in 7.5 mL of acetone. Under stirring conditions, add the drug-containing PLA solution to 7.5mL of the water phase containing 5% (w / v) stabilizer PVA to form an O / W emulsion, volatilize acetone in the fume hood for 6h, and transfer the microsphere system to 300mL Cured in water for 8h. Stand still after solidification, and after the suspended matter settles to the bottom, remove the upper aqueous solution, collect the microspheres deposited at the bottom, wash with water three times, collect the microspheres by centrifugation, and freeze-dry.
Embodiment 2
[0035] Weigh 70.8mg of triamcinolone acetonide acetate and 187.5mg of PLGA (molar ratio of lactide to glycolide=75:25, Mw=1.2wg / mol), and dissolve them in 7.5mL of dichloromethane. Under stirring conditions, add the PLGA solution containing the drug to 150mL of water phase containing 1% (w / v) stabilizer PVA to form an O / W emulsion, volatilize methylene chloride for 6h in the fume hood, and transfer the microsphere system to Cured in 300mL water for 8h. Stand still after solidification, and after the suspended matter settles to the bottom, remove the upper aqueous solution, collect the microspheres deposited at the bottom, wash with water three times, collect the microspheres by centrifugation, and freeze-dry.
Embodiment 3
[0037] Weigh 70.8mg of triamcinolone acetonide acetate and 187.5mg of PLGA (molar ratio of lactide to glycolide=75:25, Mw=1.2wg / mol), and dissolve them in 7.5mL of ethyl acetate. Under stirring conditions, add the PLGA solution containing the drug to 150mL of water phase containing 1% (w / v) stabilizer PVA to form an O / W emulsion, volatilize methylene chloride for 6h in the fume hood, and transfer the microsphere system to Cured in 300mL water for 8h. Stand still after solidification, and after the suspended matter settles to the bottom, remove the upper aqueous solution, collect the microspheres deposited at the bottom, wash with water three times, collect the microspheres by centrifugation, and freeze-dry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com