Preparation method of calcium gluconate
A technology of calcium gluconate and glucose, which is applied in the field of medicine, can solve the problems of high cost, constant synthesis yield, and long cycle, and achieve the effects of short preparation process steps, high reaction selectivity, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 5kg glucose and 5L water into a 20L reaction vessel, add about 43.4g Tempo and 77.2g phase transfer catalyst tetrabutylammonium chloride, slowly stir and drop an aqueous solution of 1.5 times the mole amount of sodium hypochlorite at room temperature, and dropwise add 1.03kg of calcium hydroxide aqueous solution, stirred at room temperature for 1h, then concentrated under reduced pressure to 1 / 2 of the original volume, finally added an equal volume of ethanol, stirred, crystallized, filtered to obtain calcium gluconate, dried under reduced pressure to obtain 5.319kg, collected The yield is 85.5%, and the purity is 99.6%.
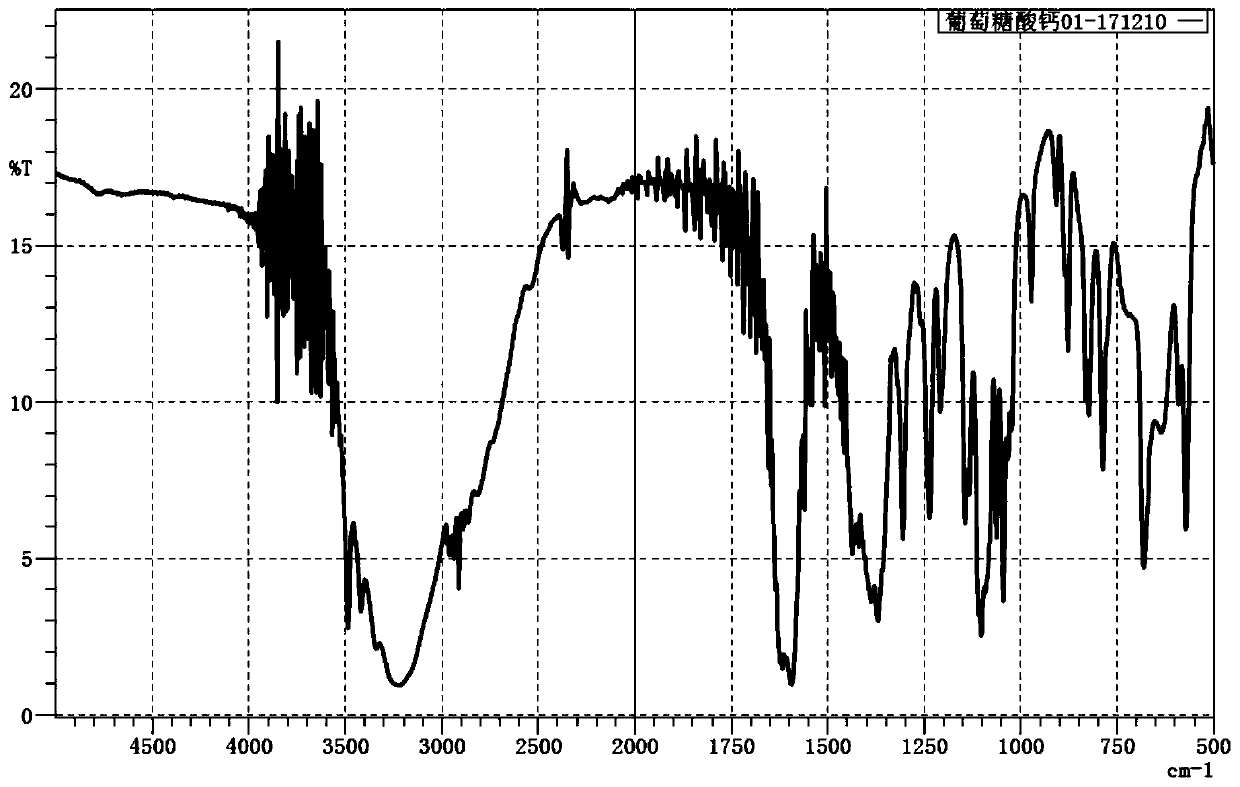
[0029] The IRAffinity-1S WL infrared tester of SHIMADZU was used to measure the infrared spectrum of the product, and the results are shown in figure 1 , verify that the product is calcium gluconate.
Embodiment 2
[0031] Add 5kg of glucose and 10L of water into a 20L reaction vessel, add about 43.4g of Tempo and 77.2g of phase transfer catalyst tetrabutylammonium chloride, slowly stir and drop an aqueous solution of 1.2 times the mole amount of sodium hypochlorite at room temperature, and dropwise add 1.03kg of calcium hydroxide aqueous solution, stirred at room temperature for 1h, then concentrated under reduced pressure to 1 / 2 of the original volume, finally added an equal volume of ethanol, stirred, crystallized, filtered to obtain calcium gluconate, dried under reduced pressure to obtain 5.114kg, and collected The yield is 82.2%, and the purity is 99.5%.
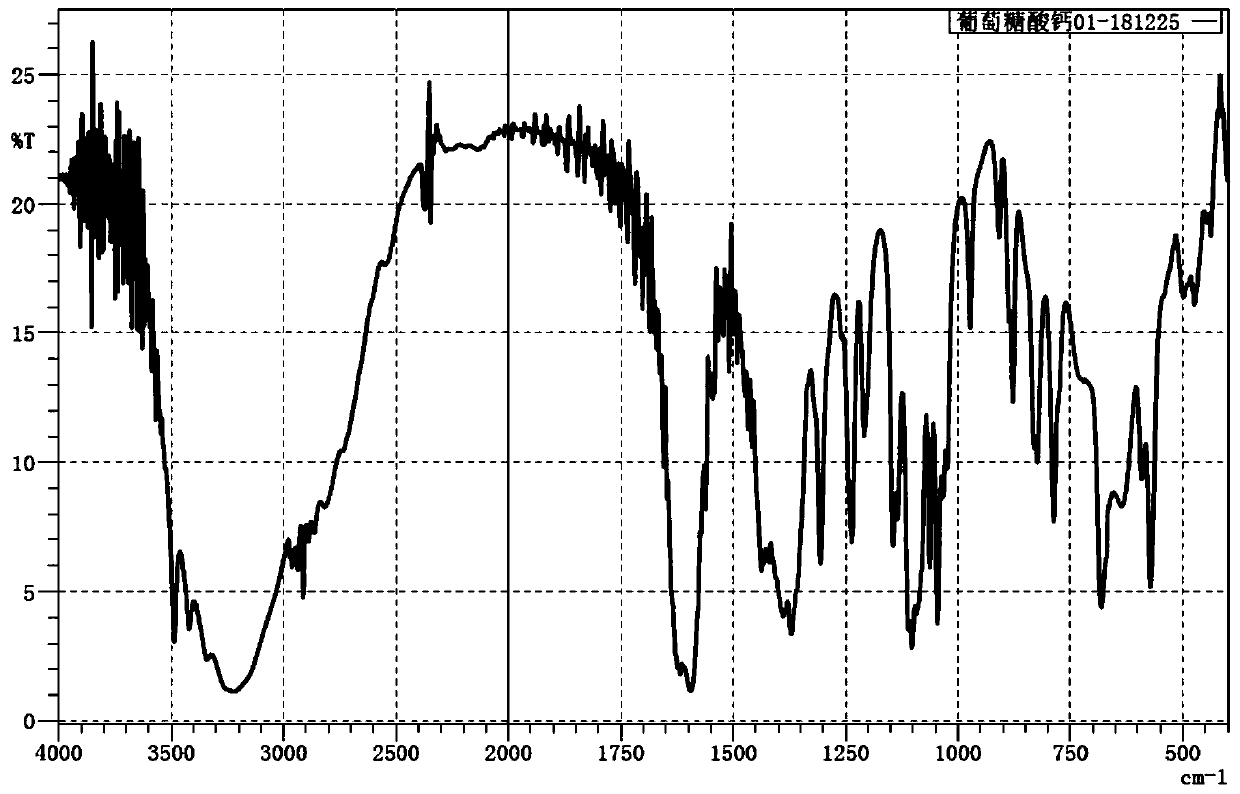
[0032] figure 2 For the infrared spectrum of the calcium gluconate prepared in Example 2, it is verified that the product is calcium gluconate.
Embodiment 3
[0034] Add 5kg of glucose and 15L of water into a 20L reaction vessel, add about 86.8g of Tempo and 77.2g of phase transfer catalyst tetrabutylammonium chloride, slowly stir and drop an aqueous solution of 1.2 times the mole amount of sodium hypochlorite at room temperature, and dropwise add 1.03 kg of calcium hydroxide aqueous solution, stirred at room temperature for 1 h, then concentrated under reduced pressure to 1 / 3 of the original volume, finally added an equal volume of ethanol, stirred, crystallized, filtered to obtain calcium gluconate, dried under reduced pressure to obtain 5.002 kg, and collected The yield is 80.9%, and the purity is 99.4%.
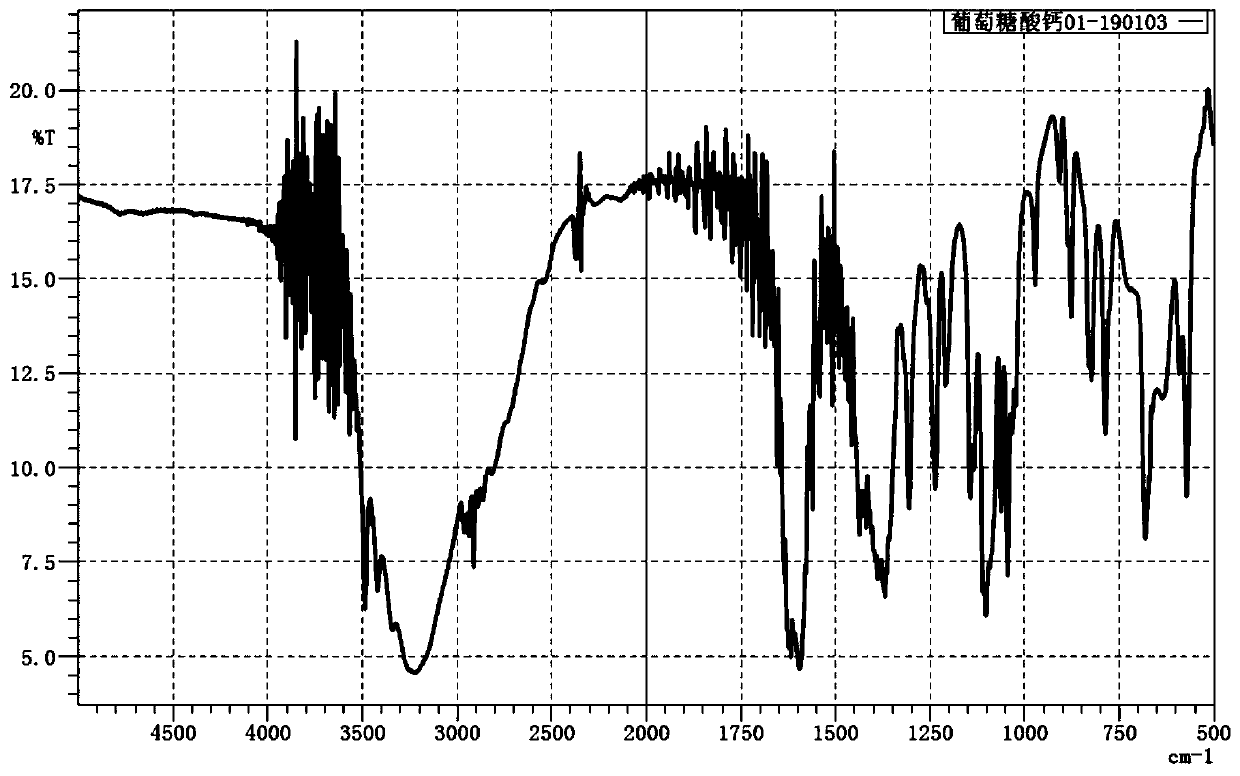
[0035] image 3 For the infrared spectrum of the calcium gluconate prepared in Example 3, it is verified that the product is calcium gluconate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com