Expression purification, crystal structure and application of mycobacterium tuberculosis ribosomal protein S7
A technology of mycobacterium tuberculosis and ribosomal protein, applied in the biological field, can solve the problems of no good treatment, large side effects, and long treatment cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1. Expression and purification of ribosomal protein S7
[0028] From tuberculosis pathogenic bacteria Mycobacterium tuberculosis H37Rv T Ribosomal protein S7, its amino acid sequence is shown in SEQ.ID.NO1, and its nucleic acid sequence is shown in SEQ.ID, NO2.
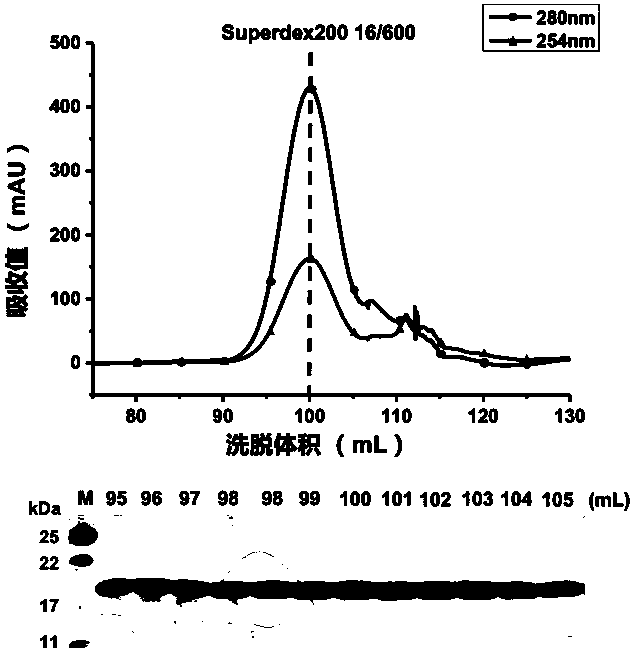
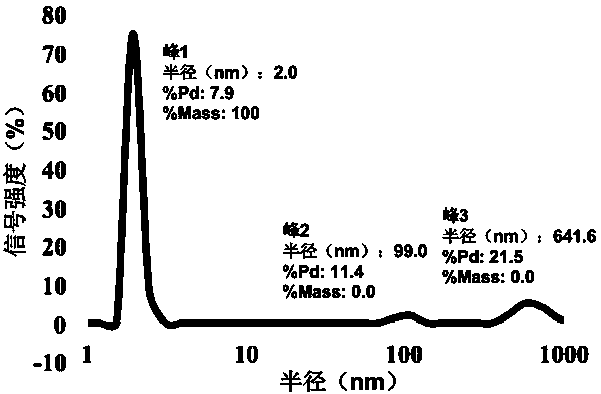
[0029] The full-length gene of mtb_rpsG was cloned into the pSMT3 vector by molecular cloning technology to express the fusion protein with 6 His and SUMO tags fused at the amino terminus, and the recombinant vector was transformed into Escherichia coli Rosetta (DE3), and cultured with shaking at 37°C When the OD600 reaches 0.6-1.0 (preferably 0.8), add IPTG with a final concentration of 0.1-1.0mM (preferably 0.4mM) to induce expression. The expression conditions are: 16°C shaking culture for 20h or 20°C shaking culture for 16h or 25°C Shake culture 8h. Preference is given to culturing at 16°C with shaking at 200rpm. Bacteria were collected by centrifugation after 20 hours for purification.
[0030]...
Embodiment 2
[0031] Example 2. Crystallization of ribosomal protein S7
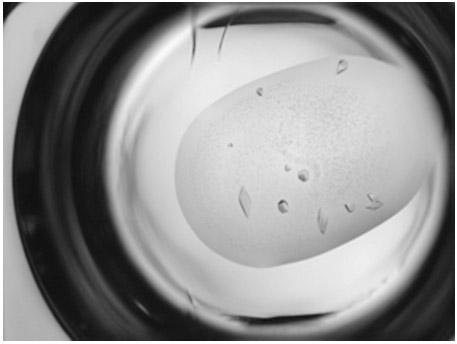
[0032] Concentrate the ribosomal protein S7 expressed and purified by the above method to a concentration of about 17 mg / ml, and use a crystallization kit (Crystal Screen Kit I / II, Index, Salt, PEG / Ion, etc. from companies such as Hampton Research) as crystal growth The initial screening conditions were carried out by hanging drop (or sitting drop) gas phase diffusion method for crystallization. The present invention obtains primary crystals under a plurality of different crystallization reagent conditions. Through the optimization and adjustment in the later stage, preferably 20% PEG3350, the buffer solution of 8% Tacsimate (pH6.0) is used as the crystal growth condition, and a set of resolution is collected. X-ray diffraction data.
Embodiment 3
[0033] Example 3. Diffraction data collection and structural analysis of ribosomal protein S7
[0034] In the present invention, the prepared crystals are treated with 25% glycerin as an antifreezing agent, and then stored in liquid nitrogen. The crystallographic X-ray diffraction data were collected at the Shanghai Light Source (SSRF) Biomacromolecular Crystallography Beamline Station (5th line and 6th station BL17U1). HKL2000 was used for data processing, and the structural analysis was based on the S7 protein (PDB ID: 1HUS) derived from the bacterium Geobacillus stearothermophilus as a model. The molecular replacement method was used, and the Refmac program and Coot software were used to make further corrections, and finally the ribosomal protein S7 was analyzed. Protein crystal structure. Those of ordinary skill in the art know that the atoms in the three-dimensional crystal structure have at least 40% of the atomic coordinates listed in Table 1, or at least 40% of the ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com