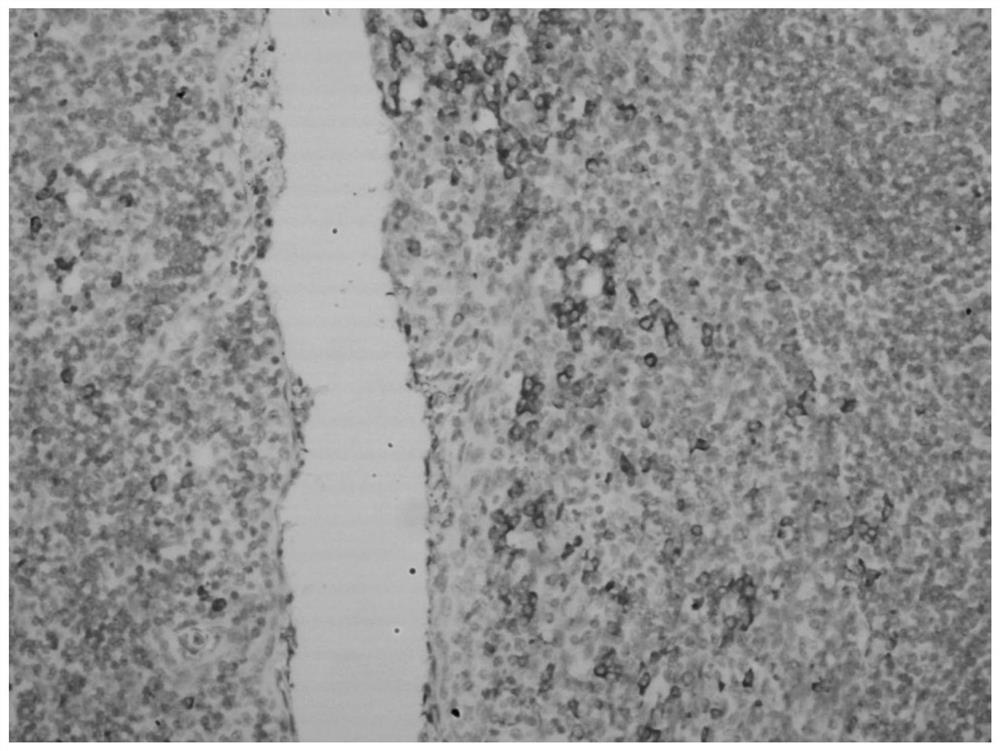
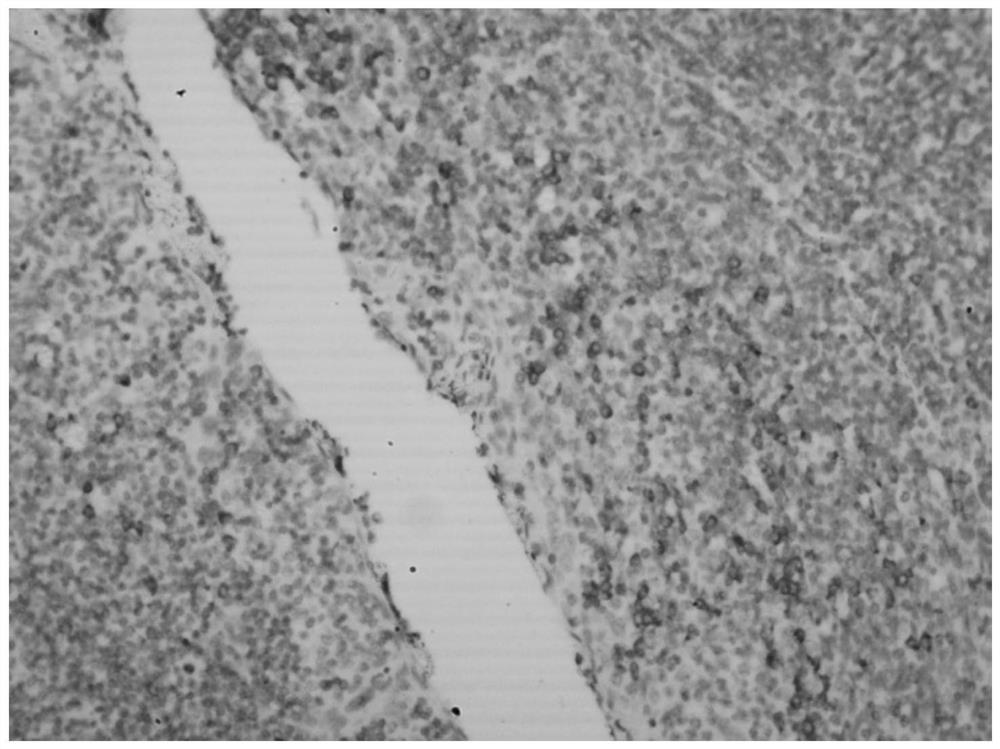
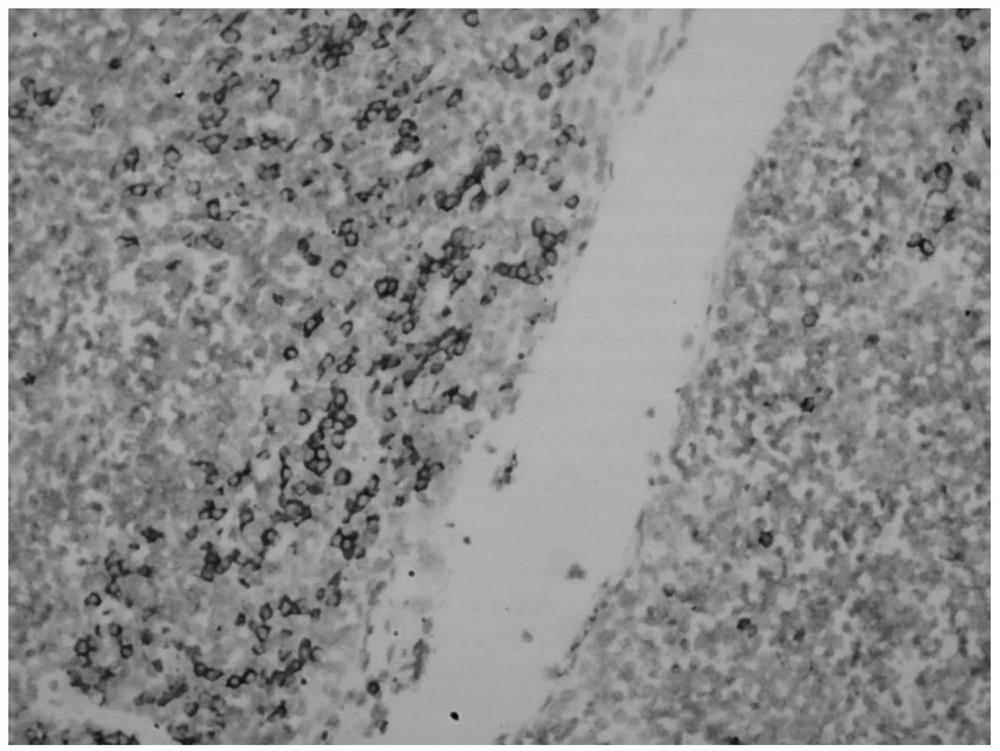
Hybridization solution for in situ hybridization, its preparation method and detection kit
A detection kit and in situ hybridization technology, applied in biochemical equipment and methods, microbiological determination/inspection, etc., can solve the problems of reducing the sensitivity of in situ hybridization, affecting clinical differential diagnosis, weak hybridization signal intensity, etc., and achieve signal and clear background, faster hybridization speed, and higher hybridization temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A method for preparing the above-mentioned hybridization solution for in situ hybridization, comprising the following steps:
[0029] (1) 1.25 g of sodium fluoride, 2.5 mL of 1 M disodium hydrogen phosphate-sodium dihydrogen phosphate buffer, 5 mL of 20-fold concentration SSC solution, 0.1 mL of 0.5 M EDTA solution, 1 mL of 50-fold concentration The concentrated Denhardts solution is mixed with 2.5 g of dextran sulfate, and the pH of the disodium hydrogen phosphate-sodium dihydrogen phosphate buffer is 7.0;
[0030] (2) centrifuging the mixed solution obtained in step (1);
[0031] (3) Add salmon sperm DNA with a final concentration of 0.1 mg / mL, and then add ultrapure water to make the volume to 50 mL.
[0032] The main mechanism of action of the hybridization solution for in situ hybridization of the present invention is now described:
[0033] Compared with the conventional hybridization solution, the hybridization solution of the in situ hybridization of the prese...
Embodiment 1
[0041] Embodiment 1: Kappa and Lambda in situ hybridization detection kit
[0042] 1. Preparation of hybridization solution (50mL):
[0043] (1) Weigh 1.25g of sodium fluoride into a 50mL centrifuge tube;
[0044] (2) Take 2.5mL of 1M disodium hydrogen phosphate-sodium dihydrogen phosphate buffer pH7.0 solution and add it to a 50mL centrifuge tube;
[0045] (3) Take 5mL of 20x SSC solution and add it to a 50mL centrifuge tube;
[0046] (4) Take 0.1mL of 0.5M EDTA solution and add it to a 50mL centrifuge tube;
[0047] (5) Take 1mL of 50x Denhardts solution and add it to a 50mL centrifuge tube;
[0048] (6) Weigh 2.5g of dextran sulfate into a 50mL centrifuge tube and mix thoroughly, and centrifuge at 1000rpm for 5 minutes;
[0049] (7) adding a final concentration of 0.1 mg / mL salmon sperm DNA;
[0050] (8) Add ultrapure water to 50mL.
[0051] Finally, the hybridization solution was used to dilute the Kappa and Lambda probes to a final concentration of 1 ng / uL.
[0052...
Embodiment 2
[0069] Example 2: HER2 fluorescence in situ hybridization
[0070] 1. Preparation of hybridization solution (50mL):
[0071] (1) Weigh 1.25g of sodium fluoride into a 50mL centrifuge tube;
[0072] (2) Take 2.5mL of 1M disodium hydrogen phosphate-sodium dihydrogen phosphate buffer pH7.0 solution and add it to a 50mL centrifuge tube;
[0073] (3) Take 5mL of 20x SSC solution and add it to a 50mL centrifuge tube;
[0074] (4) Take 0.1mL of 0.5M EDTA solution and add it to a 50mL centrifuge tube;
[0075] (5) Take 1mL of 50x Denhardts solution and add it to a 50mL centrifuge tube;
[0076] (6) Weigh 5g of dextran sulfate and add it into a 50mL centrifuge tube to fully shake and mix, and centrifuge at 1000rpm for 5 minutes;
[0077] (7) adding final concentration is 0.2mg / mL salmon sperm DNA;
[0078] (8) Add ultrapure water to 50mL.
[0079] Finally, use hybridization solution to prepare HER2 probe, including final concentration of 10ng / uL HER2 green fluorescent probe, fina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com