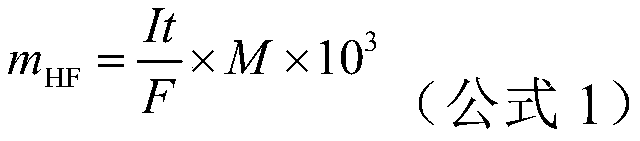Method for rapidly determining content of free acid in lithium hexafluorophosphate product in non-aqueous system
A technology for rapid determination of lithium hexafluorophosphate, which is used in measurement devices, chemical method analysis, chemical analysis by titration, etc., can solve the problems of long measurement time, poor precision, low accuracy, etc., to improve quality and shorten detection time. , the effect of improving the detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Analytical test object: Lithium hexafluorophosphate electrolyte (solid) numbered LHFP-01. The determination steps are as follows:
[0031] (1) Accurately weigh the mass in the glove box as m s (about 1.000g) lithium hexafluorophosphate electrolyte sample, place the weighed sample in a dry polytetrafluoroethylene beaker;
[0032] (2) In the transfer chamber of the glove box, add 30 mL of lithium chloride-ethanol solution with a concentration of 0.50 mol / L to the polytetrafluoroethylene beaker, and then take out the beaker;
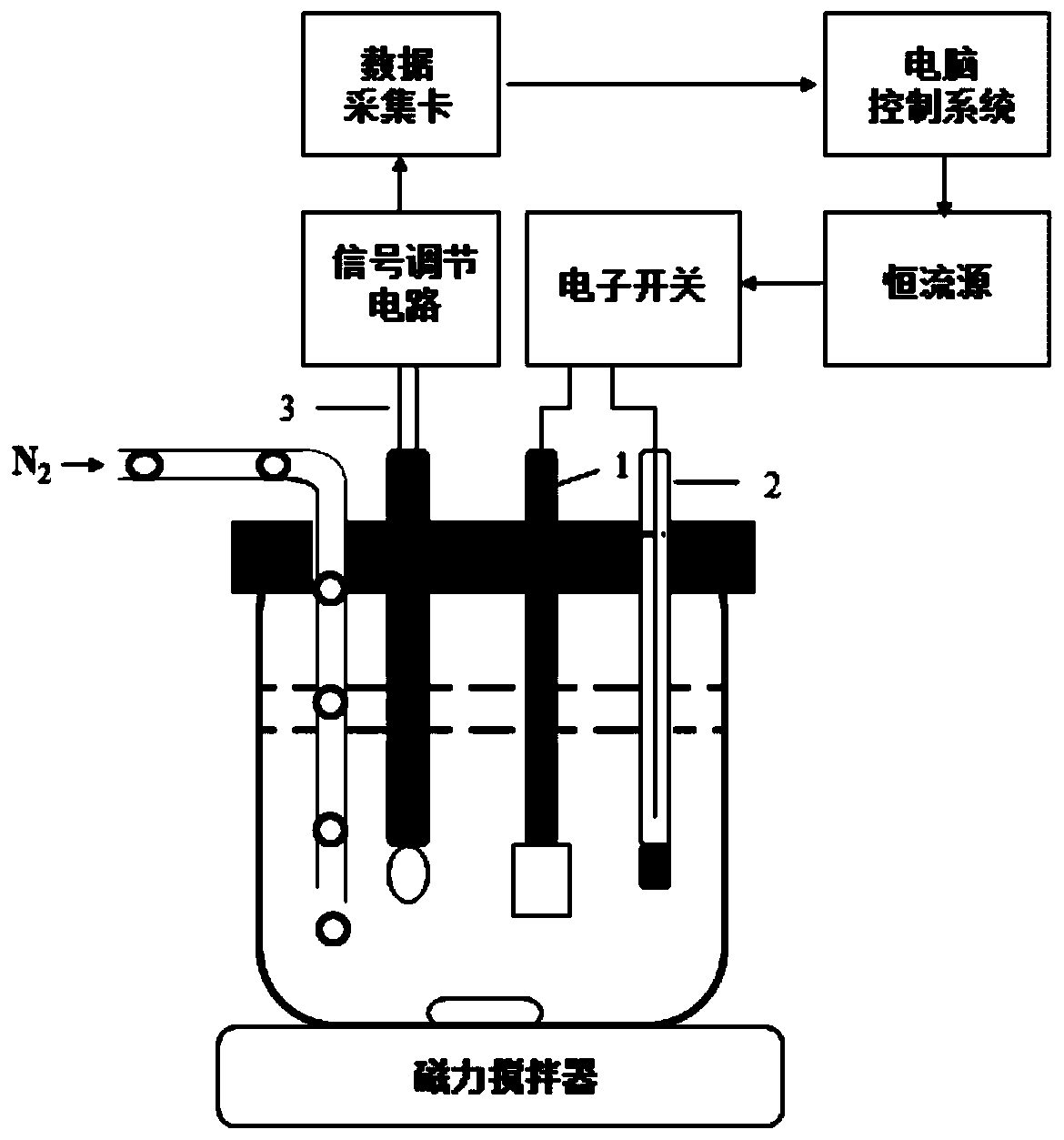
[0033] (3) Place the polytetrafluoroethylene beaker on the stirrer to stir, insert the pH indicating electrode pair and the working electrode pair, and connect the two with the acid-base coulometric titrator;
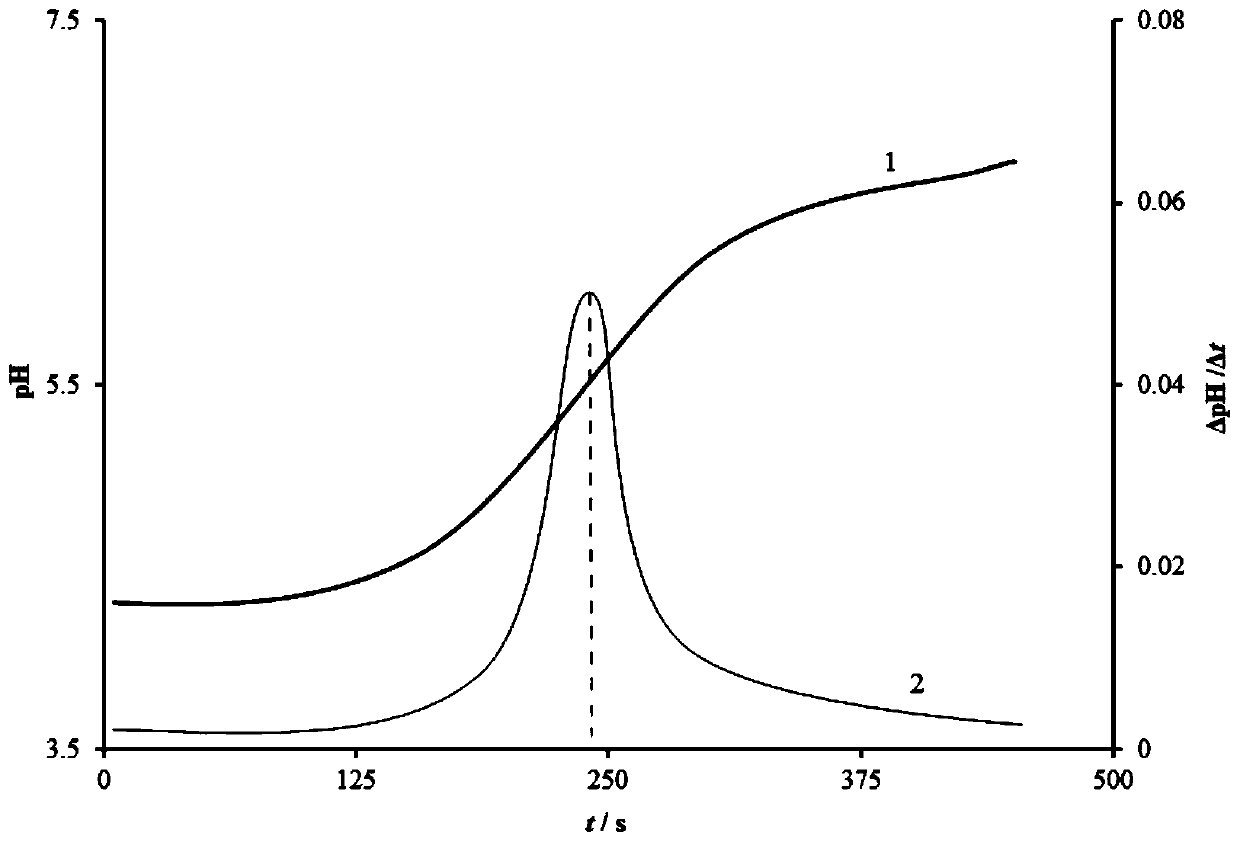
[0034] (4) After setting the electrolysis current, start the acid-base coulometric titrator, and the electrolysis will stop automatically at the titration end point, record the electrolysis time t and the electrolysis current I, and calculat...
Embodiment 2
[0036] Analysis and test object: Lithium hexafluorophosphate electrolyte solution numbered LHFP-12, which is composed of lithium hexafluorophosphate electrolyte and ethylene carbonate, with a concentration of 1mol / L. The determination steps are as follows:
[0037] (1) Add 30 mL of lithium chloride-ethanol solution with a concentration of 0.50 mol / L to a polytetrafluoroethylene beaker, place the beaker on a stirrer and stir, insert a pair of pH indicating electrodes and a pair of working electrodes, and mix the two with acid Alkaline coulometric titrator connected;
[0038] (2) Select a dry 5mL PE material syringe, take about 2mL of the lithium hexafluorophosphate electrolyte to be tested in the glove box, and seal the needle with a matching plastic sleeve. Transfer the syringe to the atmosphere and accurately weigh to 0.001g on an analytical balance;
[0039] (3) Quickly add about 1.000g of lithium hexafluorophosphate electrolyte into the above-mentioned polytetrafluoroethy...
Embodiment 3
[0042] Analysis and test object: Lithium hexafluorophosphate electrolyte solution numbered LHFP-13, which is composed of lithium hexafluorophosphate electrolyte, ethylene carbonate, dimethyl carbonate, and diethyl carbonate. The concentration of the lithium hexafluorophosphate electrolyte is 1mol / L, and the volume ratio of ethylene carbonate, dimethyl carbonate, and diethyl carbonate is 1:1:1. The assay procedure of this sample is basically the same as in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com