Preparation and application of blended nickel (II) complex with bisoxazoline derived azacyclo-carbene ligand and phosphite ester ligand
A technology of phosphite ligand and nitrogen heterocyclic carbene, which is applied in the direction of hydrocarbon production from halogen-containing organic compounds, organic compound/hydride/coordination complex catalysts, nickel organic compounds, etc., can solve the problem of inability to achieve convenient and efficient synthesis Diaryl methane compounds, inability to apply cheap and easy-to-obtain chlorinated aromatics, unfavorable staged heating or step-by-step feeding, etc., to achieve the effect of improving industrial application value, simple reaction, easy operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
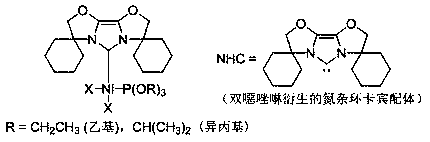
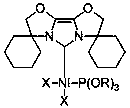
[0036] Embodiment one: Ni(NHC)[P(OR) 3 ]X 2 (R = CH 2 CH 3 , X = Br) synthesis
[0037] Under argon protection, nitrogen heterocyclic carbene NHC (0.2884 g, 1.0 mmol) was added to di(triethylphosphite) nickel(II) dibromide (0.5508 g, 1.0 mmol) in tetrahydrofuran solution, room temperature The reaction was carried out at low temperature for 3 hours, then the solvent was removed in vacuo, the residue was washed with n-hexane, the residue obtained was extracted with toluene, the clear liquid was transferred and the solvent toluene was removed to obtain an orange-yellow solid with a yield of 87%.
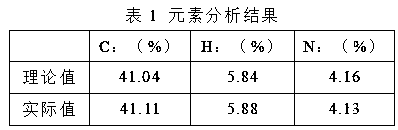
[0038] The product was subjected to elemental analysis, and the results are shown in Table 1:
[0039]
[0040] The product was characterized by NMR, and the results are as follows:
[0041] Dissolve the product in C 6 D. 6 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 HNMR (400 MHz, C 6 D. 6 ): δ 4...
Embodiment 2
[0042] Embodiment two: Ni(NHC)[P(OR) 3 ]X 2 (R = CH(CH 3 ) 2 , X = Br) synthesis
[0043] Under the protection of argon, nitrogen heterocyclic carbene NHC (0.2884 g, 1.0 mmol) was added to a tetrahydrofuran solution of di(triisopropyl phosphite)nickel(II) bromide (0.6350 g, 1.0 mmol), React at room temperature for 4 hours, remove the solvent in vacuo, wash the residue with n-hexane, extract the residue with toluene, transfer the clear liquid and remove the solvent toluene to obtain an orange solid with a yield of 82%.
[0044] Product is carried out elemental analysis, and the result is as shown in table 2:
[0045]
[0046] The product was characterized by NMR, and the results are as follows:
[0047] Dissolve the product in C 6 D. 6 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 HNMR (400 MHz, C 6 D. 6 ): δ 4.91 (s, 4H), 4.79 (dq, J = 12.0, 6.0 Hz, 3H), 2.59 – 2.49 (m, 2H), 2.31 – 2.26 ...
Embodiment 3
[0048] Embodiment three: Ni(NHC)[P(OR) 3 ]X 2 (R = CH 2 CH 3 , X = Cl) synthesis
[0049] Under argon protection, nitrogen heterocyclic carbene NHC (0.2884 g, 1.0 mmol) was added to di(triethylphosphite)nickel(II) chloride (0.4619 g, 1.0 mmol) in tetrahydrofuran solution, room temperature The reaction was carried out under vacuum for 3 hours, the solvent was removed in vacuo, the residue was washed with n-hexane, the obtained residue was extracted with tetrahydrofuran, the clear liquid was transferred and the solvent tetrahydrofuran was removed to obtain an orange-yellow solid with a yield of 80%.
[0050] Product is carried out elemental analysis, and the result is as shown in table 3:
[0051]
[0052] The product was characterized by NMR, and the results are as follows:
[0053] Dissolve the product in C 6 D. 6 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, C 6 D. 6 ): δ ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com