Preparation of a gold@graphene oxide composite nanomaterial and its application in the detection of adenosine triphosphate
A technology of graphene composite and nanomaterials, applied in the detection of adenosine triphosphate, the field of preparation of gold@graphene oxide composite nanomaterials, can solve the problem of time-consuming, difficult particle size and density control of gold nanoparticles, graphene oxide High cost and other issues, to achieve excellent properties, reduce synthesis costs, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1. Preparation of gold@graphene oxide
[0028] 1.1 Preparation of electropositive gold nanoparticles
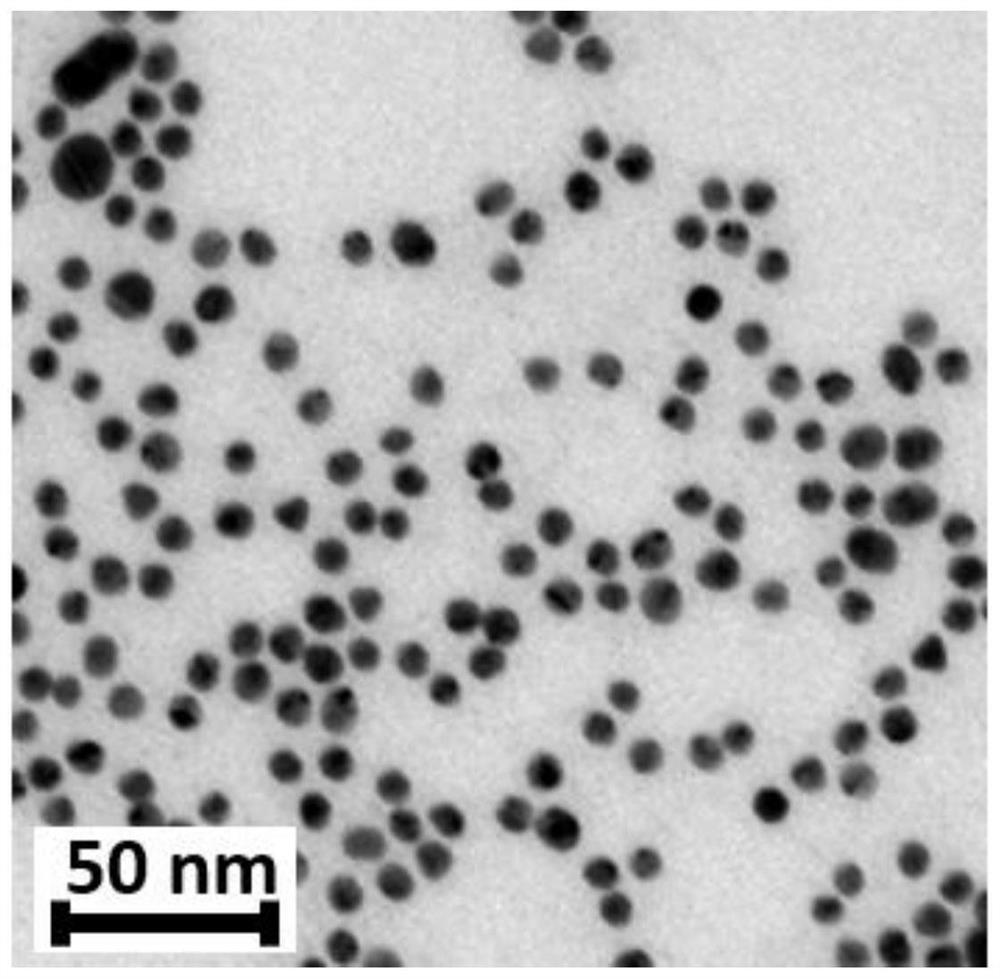
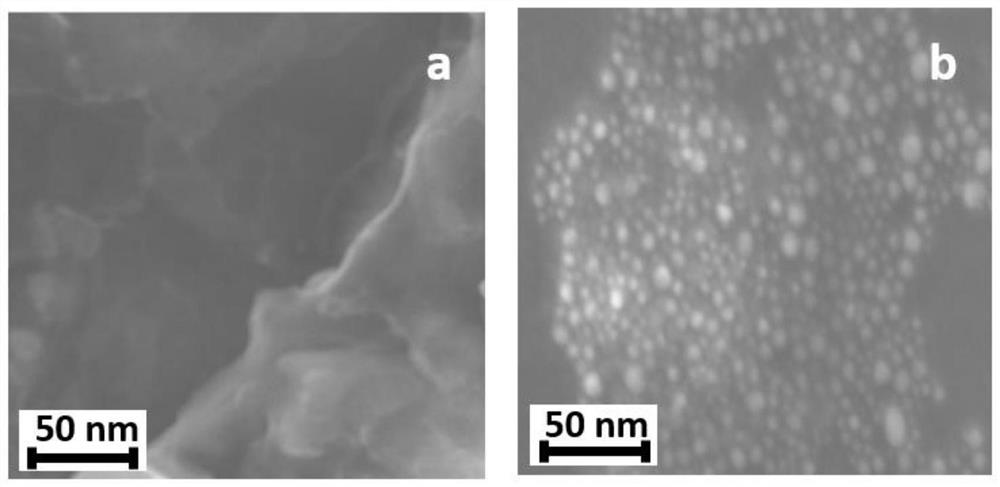
[0029] First, weigh 0.0364g of cetyltrimethylammonium bromide (CTAB) and dissolve it in 10mL of ultrapure water, and dissolve CTAB at 30°C; weigh 0.03938g of chloroauric acid and dissolve it in 10mL of ultrapure water , after all dissolved, dilute 10 times for later use; weigh 0.0378g of sodium borohydride and dissolve it in 10mL of ice-water mixture. Pipette 2 mL of CTAB solution and 15 mL of chloroauric acid solution in a glass beaker, stir at room temperature on a magnetic stirrer, start heating in a water bath after stirring for 15 minutes and gradually heat up to boiling, add 2.0 mL of sodium borohydride solution dropwise during heating, and Continue heating and stirring with magnetic force in a boiling state until the color of the solution changes to wine red, that is, positively charged gold nanoparticles are prepared. Such as figure 1 As shown, the a...
Embodiment 2
[0034] Example 2. Investigation of fluorescence quenching of gold@graphene oxide
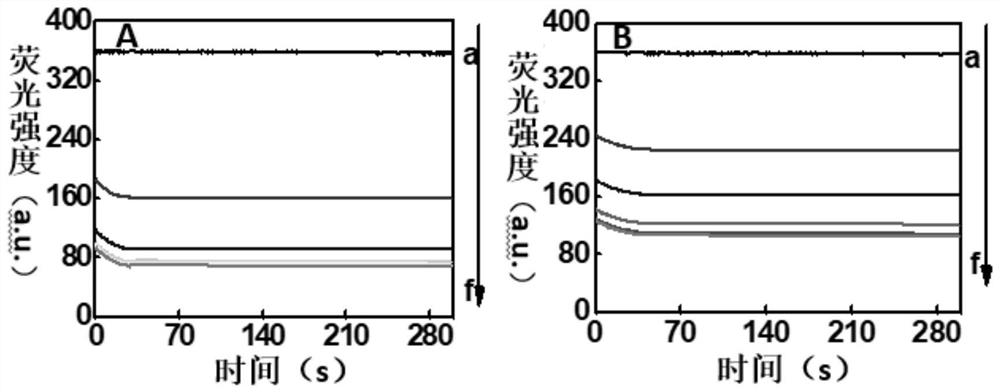
[0035] In order to prove the excellent fluorescence quenching properties of gold@graphene oxide on silver nanoclusters, the present invention establishes a time-response curve. image 3 A and image 3 B respectively investigated 0, 5, 10, 15, 20, 30 μg / mL gold@graphene oxide (a-f) and 0, 10, 20, 30, 40, 50 μg / mL graphene oxide (a-f) on 50nmol / L silver Fluorescence quenching map of nanoclusters. Such as image 3 As shown in A, when the concentration of gold@graphene oxide reaches 15 μg / mL, the maximum fluorescence quenching rate (86.7%) for silver nanoclusters is achieved, while 50 μg / mL graphene oxide can only achieve 72.2% for silver nanoclusters. % Quenching ( image 3 B), the experimental results show that the functionalization of gold nanoparticles on the surface of graphene oxide greatly increases the specific surface area, which is conducive to more adsorption of silver nanocluster pro...
Embodiment 3
[0036] Example 3. Gold@Graphene Oxide for Quantitative Detection of Adenosine Triphosphate
[0037] First, take nine 1.5mL centrifuge tubes, add 10 μL of silver nanoclusters with a concentration of 10 μmol / L, 6 μL of gold@graphene oxide with a concentration of 1.0 mg / mL, and 10 μL of adenosine triphosphate ( The final concentrations were 0, 5.0pmol / L, 50pmol / L, 200pmol / L, 500pmol / L, 1.0nmol / L, 1.5nmol / L, 2.5nmol / L and 5.0nmol / L), and diluted with Tris buffer to The final volume was 400 μL. After incubation at room temperature for 40 minutes, 20 U of nuclease was added and incubated at 37° C. for 60 minutes to detect the recovery of the fluorescence of the silver nanoclusters. Such as Figure 4 As shown in A, the fluorescence recovery of silver nanoclusters has a good proportional relationship with the concentration of adenosine triphosphate, and has a good linear relationship with the concentration of adenosine triphosphate in the range of 5.0pmol / L to 2.5nmol / L, and the corr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com