Rhodol derivative dye and application thereof
A technology of derivatives and dyes, applied in the directions of organic dyes, styryl dyes, methine/polymethine dyes, etc., can solve the problems of high photostability, low contrast ratio of probe imaging, and low quantum yield, etc. The effect of excellent photophysical properties and excellent photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Design and Synthesis of Rhodol Derivative Dyes--Rhodol-NIR.
[0058] Freshly distilled cyclohexanone (3.3 mL, 31.9 mmol) was added dropwise to concentrated H 2 SO 4 (35 mL) and the mixture was cooled to 0 °C. Then, 4-diethylaminoketoacid (16 mmol) was added portionwise with stirring. After reacting at 90°C for 1.5 hours, the mixture was poured into ice (150 g). Perchloric acid (70%, 3.5 mL) was then added, and the resulting precipitate was filtered and washed with cold water (50 mL) to give 9-(2-carboxyphenyl)-6-(diethylamino)-1,2,3, 4-Tetrahydrooxyalkyl (2a), using 9-(2-carboxyphenyl)-6-(diethylamino)-1,2,3,4-tetrahydrooxyalkyl (237.6mg, 0.5mmol ) and 3,5-difluoro-4-hydroxybenzaldehyde (118.5mg, 0.75mmol), refluxed in acetic acid at a high temperature of 110°C, and spin-dried through a silica gel column to obtain Rhodol-NIR as a blue solid (89.2mg, yield 29%), the specific synthesis process is as follows:
[0059]
[0060] The structural formula of Rhodol-NIR ...
Embodiment 2
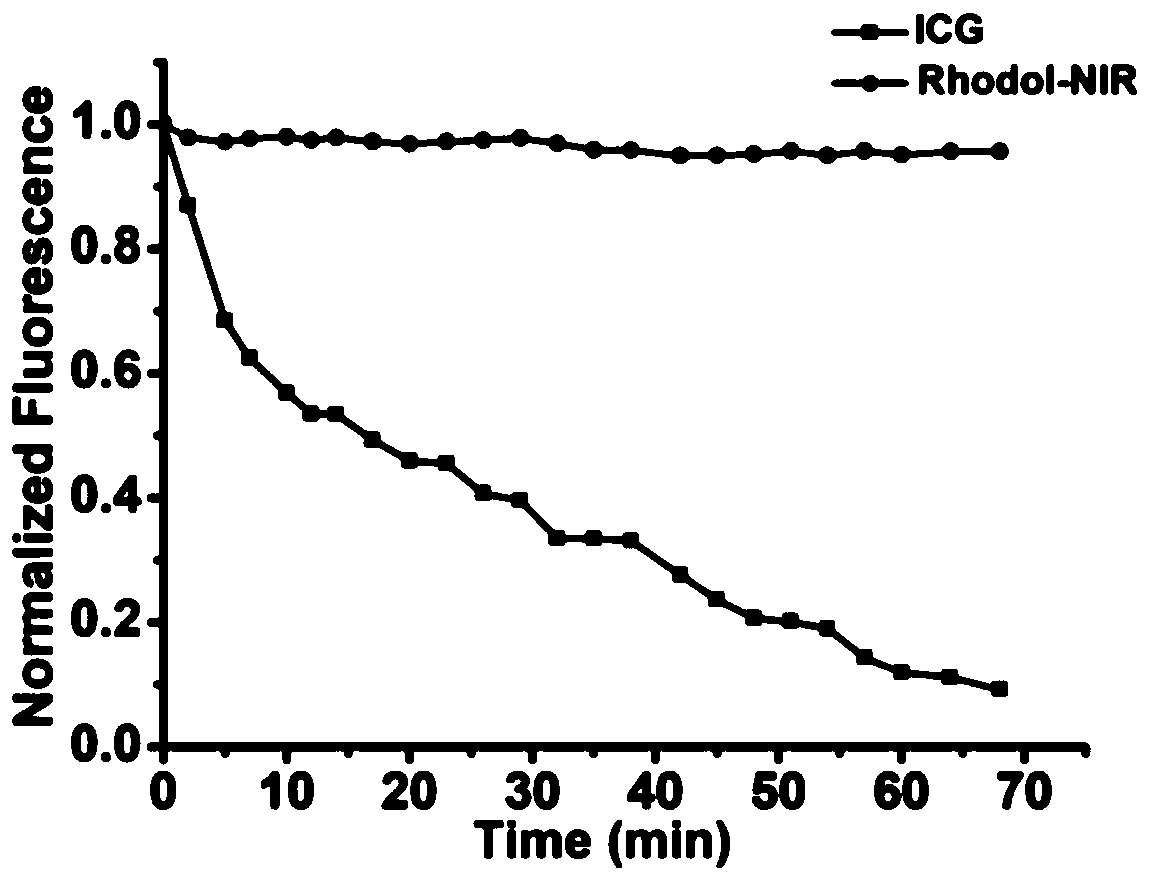
[0064] Photophysical Properties of Rhodol-NIR
[0065] Steps:
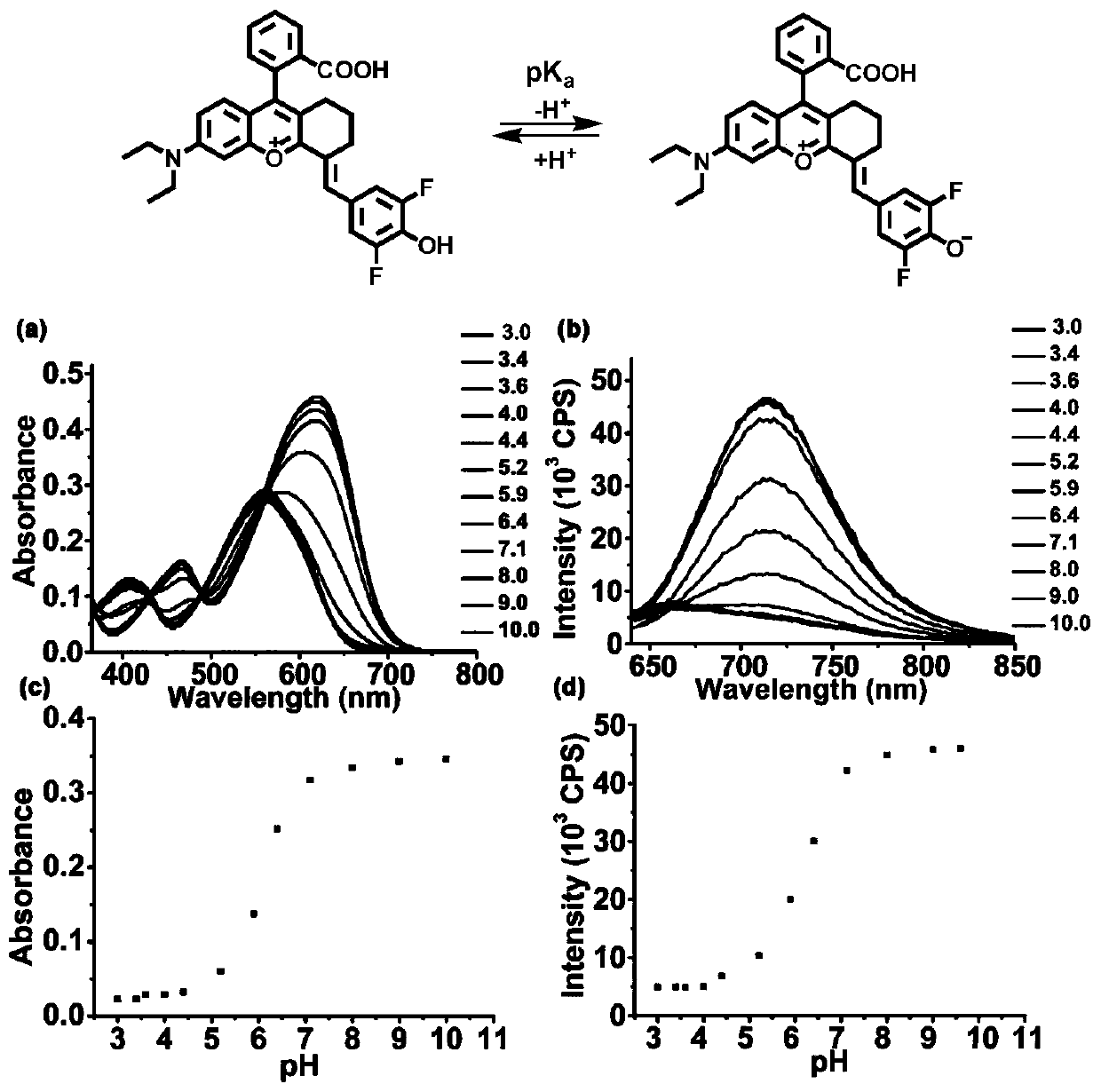
[0066] In order to determine the pKa value of Rhodol-NIR, use NaOH and hydrochloric acid to adjust the pH, prepare phosphate buffer solution containing 0.5% DMSO as a co-solvent and have different pH values, mix Rhodol-NIR (15 μ M) with different buffer systems, use The absorption spectrum of Rhodol-NIR in different pH buffer systems was measured by UV-1800 spectrophotometer, and the fluorescence spectrum of Rhodol-NIR in different pH buffer systems was measured by FS5 fluorometer, and the excitation wavelength was 620nm. The pH curve was then plotted using absorbance at 650 nm and fluorescence intensity at 720 nm. Calculate the pKa of the compound according to the Henderson-Hasselbach equation, the specific results are as follows figure 1 shown.
[0067] Rhodol-NIR has an absorption maximum at 630nm, but it produces a significantly enhanced absorption band in the broad NIR region from 620nm to 700nm. from fi...
Embodiment 3
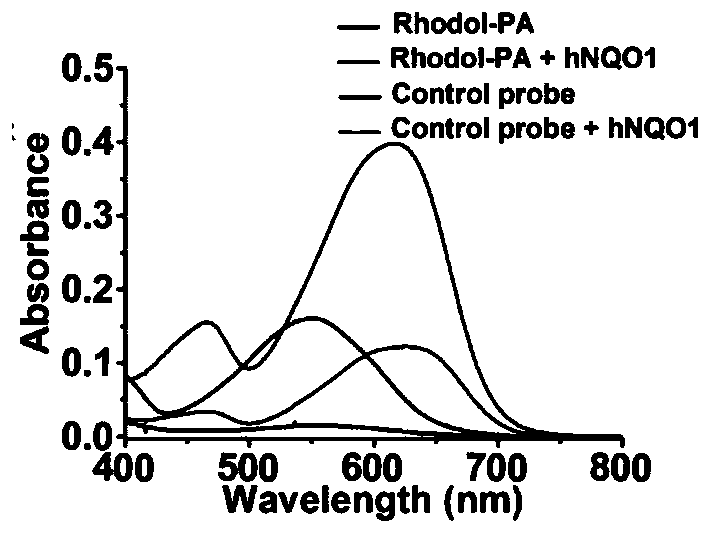
[0077] Design and Synthesis of PA Probe Based on Rhodol-NIR
[0078] The specific synthesis process is:
[0079] B. Synthesis of trimethyl-locked quinonepropionic acid: 3,3-dimethacrylate (1.60mL, 12mmol) was added to 2,3,5-trimethyl-1,4-benzenediol (1.52 g, 10 mmol) and methanesulfonic acid (15 mL), the mixture was stirred at 70°C for 2 hours. After cooling to room temperature, the reaction mixture was diluted with water to 150 mL and extracted three times with 70 mL of dichloromethane. Extract with saturated NaHCO 3 solution and NaCl solution, washed with anhydrous Na 2 SO 4 Dry and concentrate in vacuo. with 30% CHCl 3 Recrystallization in petroleum ether afforded 6-hydroxy-3,3,5,7,8-pentamethylcyclolactone-2-one as a white solid (1.85 g, 79.2% yield).
[0080]To a solution of 6-hydroxy-3,3,5,7,8-pentamethylcyclic lactone-2-one (1.17 g, 5 mmol) in acetonitrile (60 mL) and water (25 mL) was added NBS (0.98 g, mmol). The reaction mixture was then stirred at room temp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com