Brush copolymer and preparation method thereof
A copolymer and brush-shaped technology, which is applied in the field of preparation of brush-shaped copolymers, can solve problems such as poor uniformity of brush-shaped polymers, unclear structural components, and limited application development, and achieve good uniformity, clear structure and composition , good control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
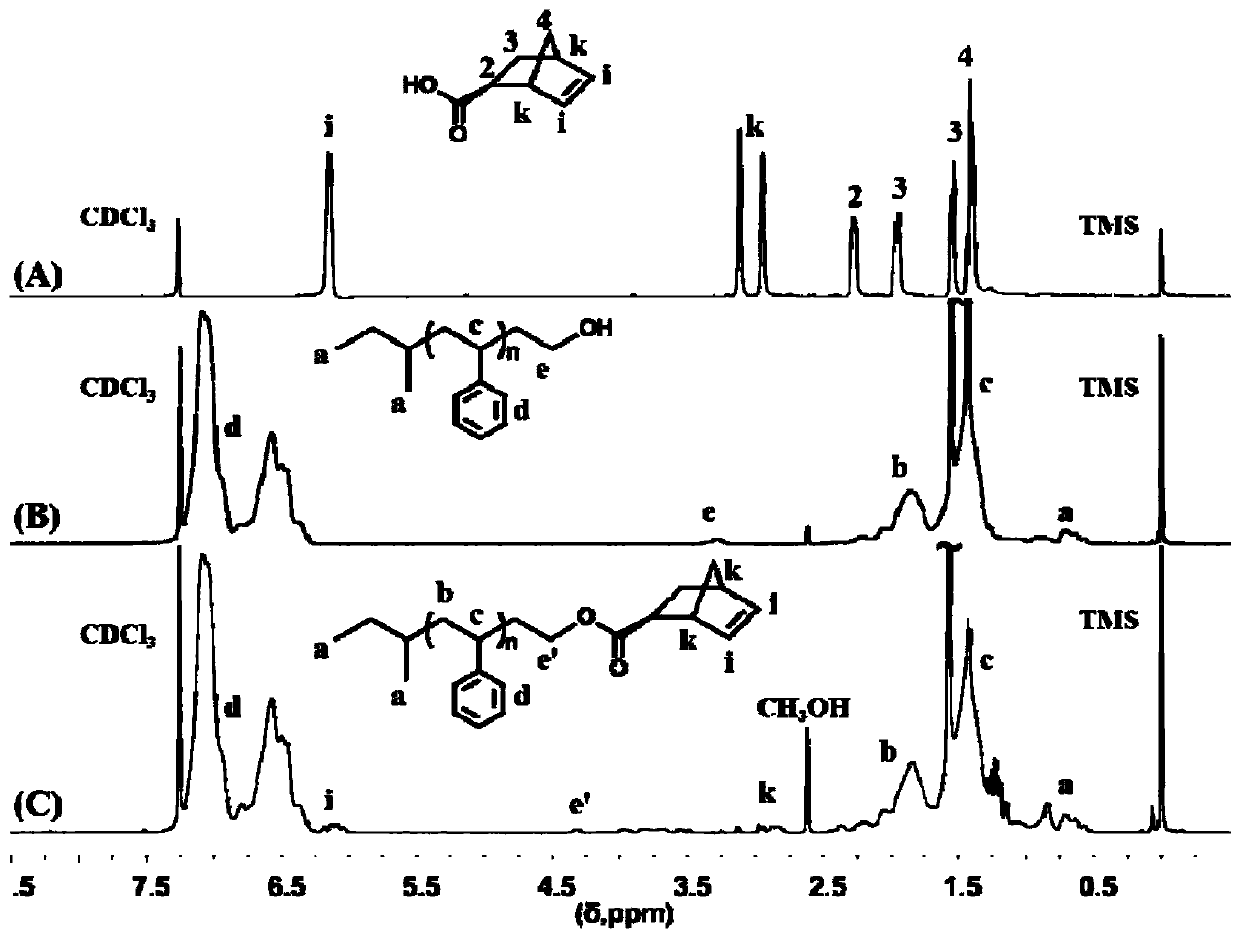
[0097] Example 1: Preparation of a brush-shaped copolymer containing polystyrene and polyisoprene
[0098] (1) Preparation of hydroxyl-terminated polystyrene (PS-OH)
[0099] With sec-butyllithium ( sec -BuLi, 1.0 mL, 1.32 mmol) was used as the initiator and benzene (50 mL) was used as the solvent to initiate the living anion polymerization of styrene (8.4 mL, 73.3 mmol) at 25 °C. After 8 hours of reaction, ethylene oxide was added (3.0 mL, 59.4 mmol) for another 0.5 h and terminated with methanol.
[0100] After the reaction, the reaction solution was concentrated by rotary evaporation, precipitated in methanol three times, and then dried in a vacuum oven at 40°C for 24 hours to obtain a white solid powder, which was hydroxyl-terminated polystyrene PS-OH, with a yield of 99%.
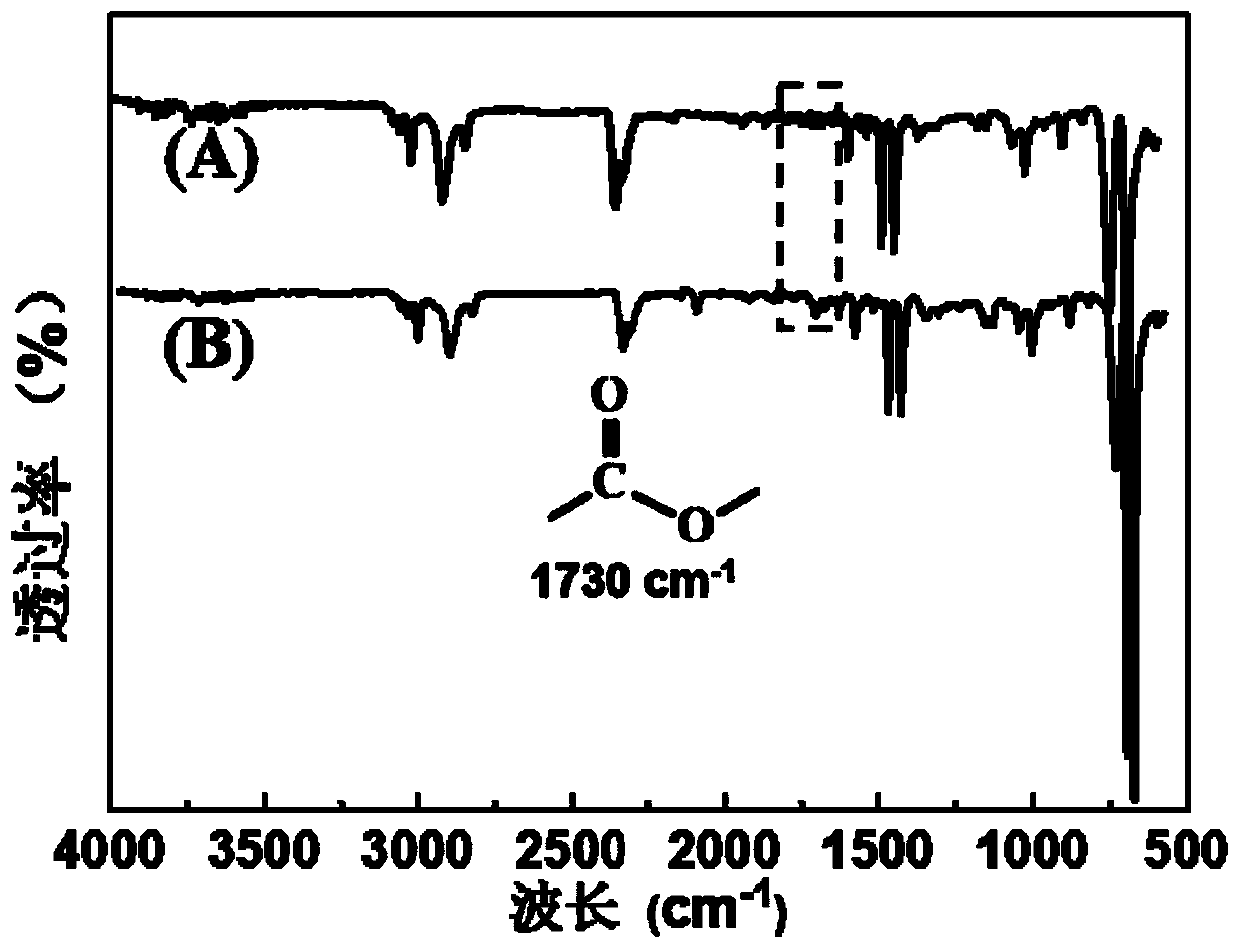
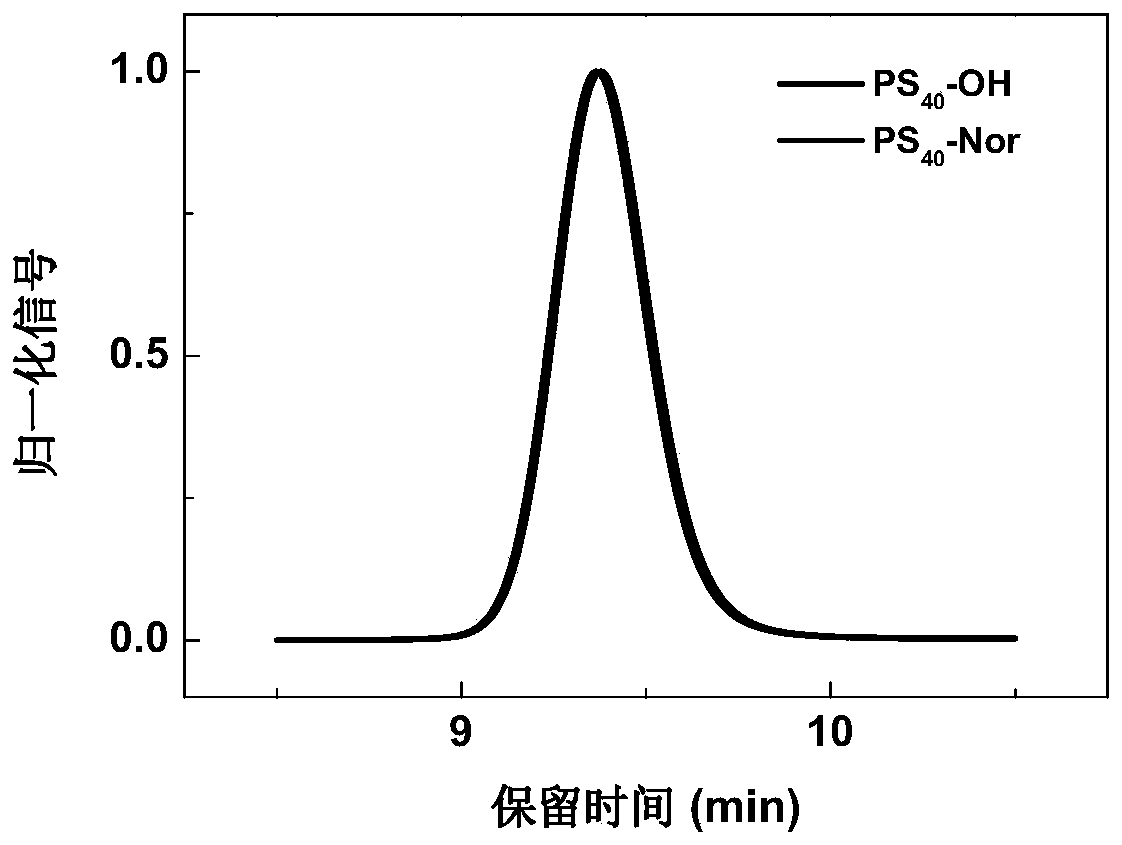
[0101] (2) Preparation of norbornene-terminated polystyrene (PS-Nor)
[0102] Weigh PS-OH (2.97 g, 0.74 mmol) and N,N’-diisopropylcarbodiimide (1.53 g, 12.15 mmol), CH 2 Cl 2 (30 mL) was dissolve...
Embodiment 2
[0120] Example 2: Preparation of amphiphilic brush copolymer containing polystyrene and polyethylene glycol
[0121] (1) Preparation of norbornene-terminated polyethylene glycol (PEG-Nor)
[0122] Put the 100 mL branch flask (including stirring bar) and the constant pressure dropping funnel into a blast drying oven at 120°C to dry for 6 h, take them out and put them in a desiccator to cool to room temperature, and then put the whole set of glass devices into the double row Tube. Weigh PEG-OH (1.55 g, 0.78 mmol) and N,N’-diisopropylcarbodiimide (1.57 g, 12.42 mmol) in 30 mL of anhydrous CH 2 Cl 2 to ensure complete dissolution. in N 2 Under the protection of the atmosphere, quickly inject into the branched flask, and continue to N 2 Atmosphere protection. weigh exo - 5-Norbornene-2-carboxylic acid (0.54 g, 3.88 mmol) and 4-(dimethylamino)pyridine (0.17 g, 1.37 mmol) were dissolved in 20 mL of anhydrous CH 2 Cl 2 to ensure complete dissolution. in N 2 Under the protec...
Embodiment 3
[0131] Example 3: Preparation of polymer micelles loaded with hydrophobic doxorubicin molecules by dialysis
[0132] Take the amphiphilic brush copolymer P (PS) prepared by embodiment two 35 - co -P(PEG) 15 (10 mg) into a round bottom flask, add 2 mL tetrahydrofuran, measure 0.2 mL anticancer drug doxorubicin (DOX) in dimethyl sulfoxide (DMSO) solution (5 mg·mL -1 ) into the tetrahydrofuran solution, under continuous stirring, use a micro-injector at a slow speed (1 mL·h -1 ) was added deionized water (2 mL), after the injection was complete, stirred for 12 hours, and transferred to the -1 Dialyze in a dialysis bag for 24 hours under agitation. The volume of the dialyzed solution was adjusted to 10 mL to obtain polymer micelles loaded with hydrophobic doxorubicin molecules. The morphology and particle size of the polymer micelles were characterized by transmission electron microscopy (TEM) and dynamic light scattering (DLS). Figure 25 The transmission electron microgra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com