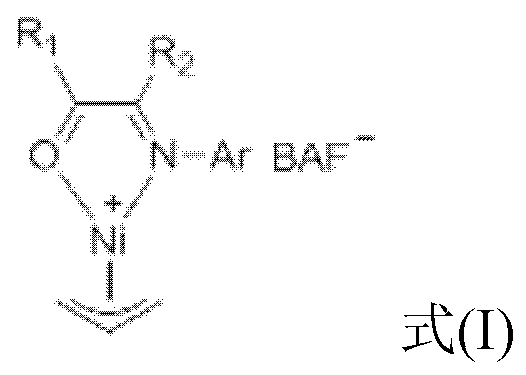
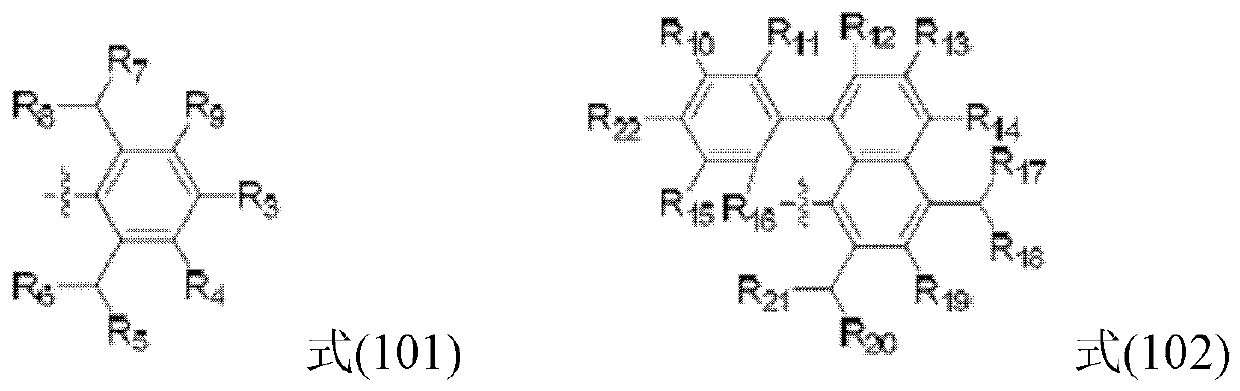
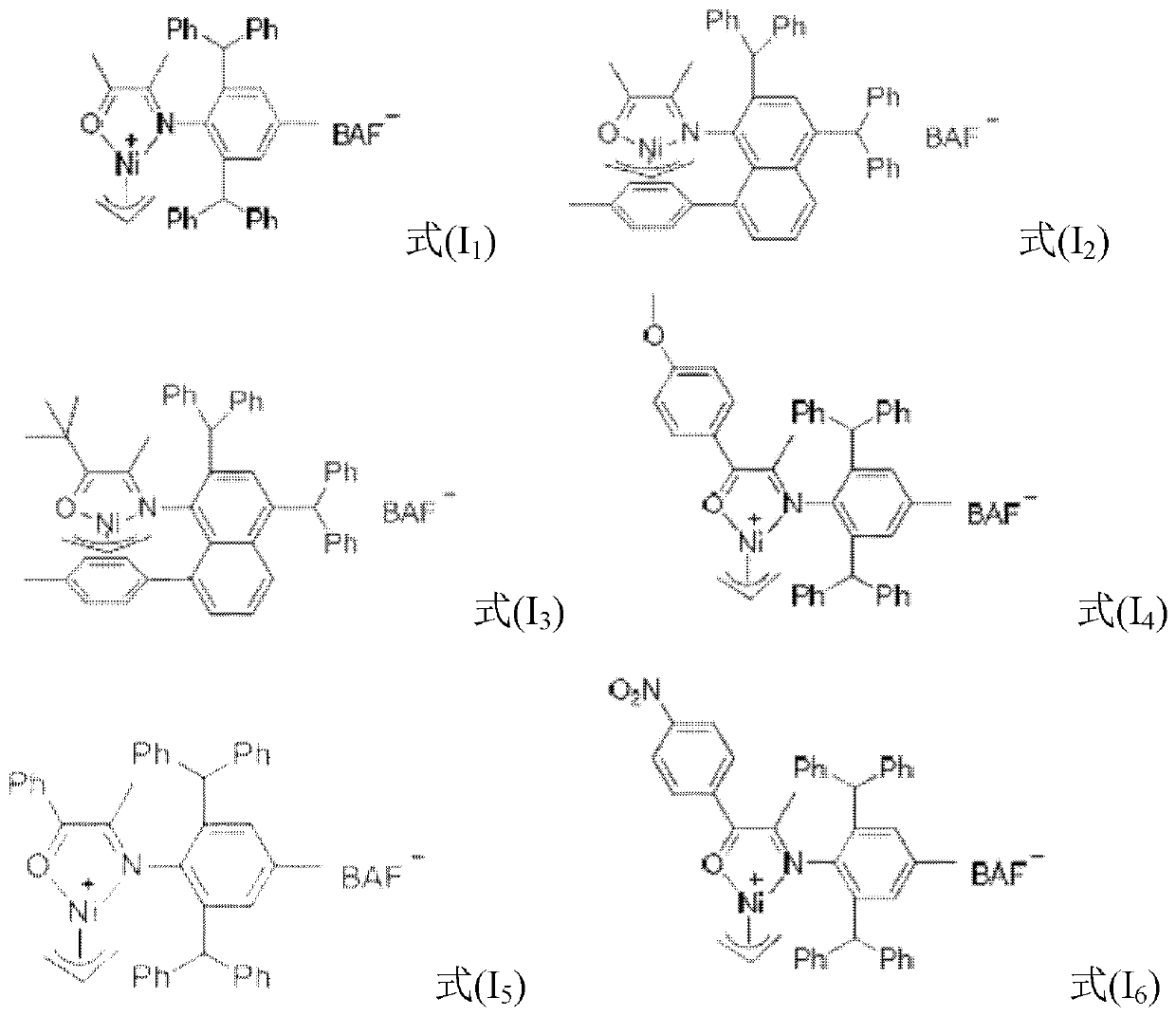
Large-steric-hindrance ketimine nickel catalyst as well as ligand compound, preparation method and application thereof
A technology of ketimine nickel and complexes, which is applied in the field of preparation of large sterically hindered ketimine nickel catalysts and ligand compounds thereof, can solve the problems of low activity and low molecular weight of polyethylene products, and achieves increased molecular weight and improved thermal conductivity. Effects of stability and chemical activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: 3-((2,4-bis(xylylmethyl)-8-(p-tolyl)naphthalen-1-yl)imino)butan-2-one (II 1 ) preparation
[0069]
[0070] Under nitrogen protection, 2,4-benzhydryl-8-(p-tolyl)naphthalene-1-amine (1.13 g, 2 mmol), 2,3-butanedione (344 mg, 4 mmol) and p-toluenesulfonic acid (20 mg) in toluene (20 ml) were stirred at 80°C for 24 hours and passed through a thin The reaction was monitored by layer chromatography (TLC). The reaction was terminated after showing a major product spot on the TLC plate. The solvent was evaporated under reduced pressure, and the remaining mixture was diluted in methanol (30 mL) and stirred for 1 hour. A yellow solid (887 mg, 70% yield) was isolated by vacuum filtration. The obtained product was detected and analyzed, and the results confirmed that it was the title compound.
[0071] 1 H NMR (400MHz, CDCl 3 )δ8.00(d, J=8.3Hz, 1H), 7.38-7.32(m, 1H), 7.24-6.97(m, 21H), 6.90-6.82(m, 2H), 6.76(d, J=6.6Hz , 2H), 6.65(s, 1H), 6.21(s, 1H, CHPh 2 ...
Embodiment 2
[0074] Example 2: 4-((2,4-bis(xylylmethyl)-8-(p-tolyl)naphthalene-1-yl)imino)-2,2-dimethylpenta-3- Ketone (II 2 ) preparation
[0075]
[0076] Under nitrogen protection, in a 250 ml round bottom flask equipped with reflux condenser, magnetic stirring device and oil bath, at 30 °C, 1-tert-butyl-1-propyne (960 mg, 10 mmol) , 0.01 equivalent of ruthenium trichloride (20.7 mg, 0.1 mmol) and 3 equivalents of iodobenzenediacetic acid (9660 mg, 30 mmol) in dichloromethane (40 ml) and water (10 ml) mixed solution was stirred for 5 Hour. The mixture was then separated using a separatory funnel, the dichloromethane phase was dried over anhydrous sodium sulfate, filtered and the solvent was removed in vacuo. The crude product was purified by standard silica gel chromatography using hexane / ethyl acetate as eluent to afford 4,4-dimethylpentane-2,3-dione. Since 4,4-dimethylpentane-2,3-dione has a low boiling point, it can be used directly in the next step.
[0077] Next, 2,4-benzhy...
Embodiment 3
[0081] Example 3: 2-((2,6-bis(xylylmethyl)-4-methylphenyl)imino)-1-(4-methoxyphenyl)propan-1-one ( II 3 ) preparation
[0082]
[0083] Under nitrogen protection, 2,6-bis(diphenylmethyl)-4-methylaniline (879 mg, 2 mmol), 1-(4-methoxyphenyl)propane-1,2-dione (712 mg, 4 mmol) and p-toluenesulfonic acid (20 mg) in toluene (20 ml) in Stir at 120° C. for 48 hours until there is a main product spot on the TLC plate, then the reaction is terminated. The solvent was evaporated under reduced pressure, and the remaining mixture was diluted in methanol (30 mL) and stirred for 1 hour. A yellow solid (720 mg, 60% yield) was isolated by vacuum filtration. The obtained product was detected and analyzed, and the results confirmed that it was the title compound.
[0084] 1 H NMR (400MHz, CDCl 3 )δ7.90(d, J=8.8Hz, 2H), 7.32-7.16(m, 13H), 7.04(dd, J=10.9, 7.7Hz, 8H), 6.74(d, J=8.8Hz, 2H), 6.67(s, 2H), 5.31(s, 2H, CHPh 2 ), 3.84(s, 3H, OMe), 2.15(s, 3H, aryl-CH 3 ), 1.08 (s, 3H, N=CM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com