Preparation method and application of tumor-targeted nano-artificial antibody
An artificial antibody and targeting technology, applied in the direction of nanotechnology, medical preparations of non-active ingredients, pharmaceutical formulas, etc., can solve the problems of large batch performance differences, immunogenicity, long screening cycle, etc., and achieve long-term use Lifespan, strong resistance to harsh environments, and the effect of shortening the screening time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of nano-gold spheres and nano-gold rods
[0038] 1. Preparation of gold nanospheres
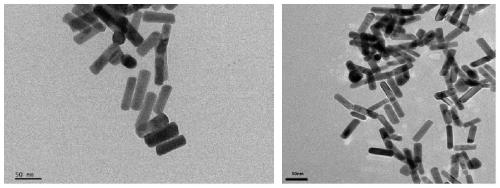
[0039] 0.0255g HAuCl 4 Dissolve in 260mL of water, heat to boil, then add 15g of 1% sodium citrate solution to the solution. After the color of the solution remains unchanged, continue to boil for 15min, centrifuge to collect the gold nanospheres, and store them for later use. The particle size and morphology of the synthesized gold nanoparticles were studied by transmission electron microscopy (TEM), such as figure 1 shown.
[0040] 2. Preparation of gold nanorods
[0041] 0.3553 g of CTAB, 0.25 mL of 0.1 M HAuCl 4 and 0.2277 mg of NaBH 4 Mix rapidly for 2 minutes, and the solution turns brown after the mixing is completed. This is the seed solution, which was left to stand at 28°C for 2 hours. Then sequentially add 15mL 0.1M HAuCl 4 , 5.096gAgNO 3, 0.55mL HCl and 42.2712mg ascorbic acid were added to 300mL solution containing 10.9535g CTAB, and then 1....
Embodiment 2
[0042] Example 2: Preparation of Nano-artificial Antibodies Targeting Epidermal Growth Factor Receptor (EGFR)
[0043] 1. Design and synthesis of nanoparticles
[0044] N-isopropylacrylamide (58-X mol%), charged functional monomer (3-acrylamidopropyl) trimethylammonium chloride (X mol%), N-tert-butylacrylamide (35mol %), the crosslinker N,N'-methylenebisacrylamide (7 mol%) and sodium dodecylsulfonate (10 mg) were dissolved in water to give a total monomer concentration of 130 mM. After adding the initiator, polymerization was carried out at 65° C. for 3 hours with a magnetic stirrer under a nitrogen atmosphere. The polymerized solution was purified by dialysis against an excess of pure water, and polymer nanoparticles were obtained after freeze-drying.
[0045] 2. Preliminary screening of artificial antibodies
[0046] The epidermal growth factor receptor (EGFR) was selected as the target, and the molecularly imprinted polymer nanoparticles were used as the biomimetic antib...
Embodiment 3
[0049] Example 3: Preparation of Nano-artificial Antibodies Targeting Epithelial Cell Adhesion Molecule (EpCAM)
[0050] Dissolve 228.6 mg of N-isopropylacrylamide, 23 mg of acrylamide, 82.6 mg of N-tert-butylacrylamide, 50 mg of N,N'-methylenebisacrylamide and sodium dodecylsulfonate SDS (30 mg) In 50mL of water, the total monomer concentration was 65mM, and 2mg of epithelial cell adhesion molecule (EpCAM) polypeptide was added at the same time, the mixed solution was filtered, nitrogen gas was blown for 30min, ammonium persulfate was added, and reacted at 65°C for 10h. The polypeptide template was then eluted with 1 M sodium chloride solution to obtain nanoartificial antibodies against epithelial cell adhesion molecule (EpCAM). Then the reaction mixture was dialyzed for 3 days, and then freeze-dried to obtain the nano-artificial antibody.
[0051] The scanning electron microscope image of the nano-artificial antibody obtained by mixing the above monomer solution and gold na...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com