Method for analyzing genotoxic impurities in pantoprazole sodium and starting raw materials of pantoprazole sodium
A technology for pantoprazole sodium and genotoxicity, applied in the directions of material separation, analysis materials, measuring devices, etc., can solve the problems of separation and determination methods that have no public reports, etc., achieve high accuracy, simple and convenient experimental operation method, strong specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
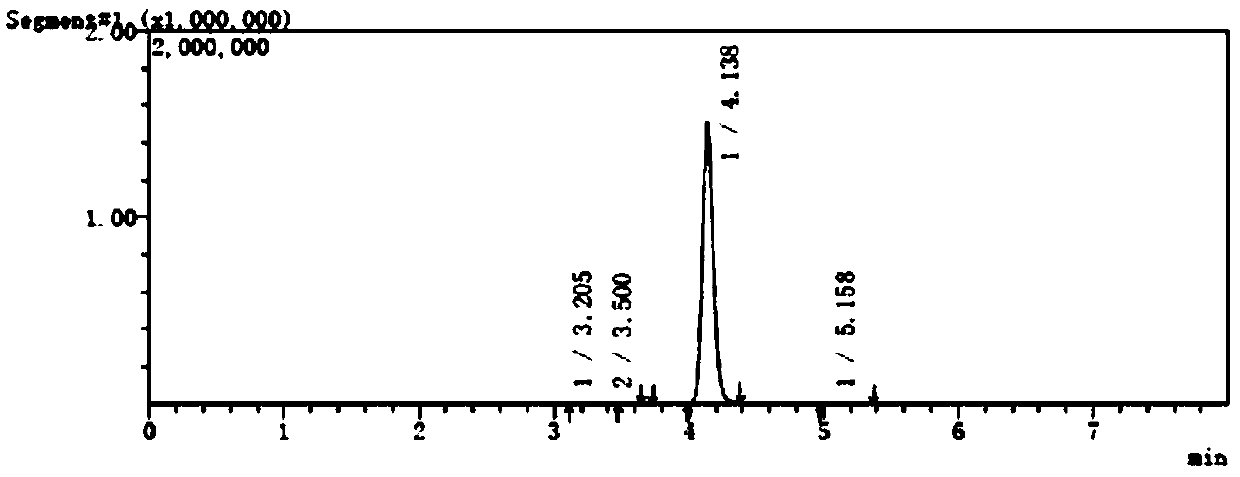
[0059] The separation and determination method of potential genotoxic impurities in embodiment 1, pantoprazole sodium
[0060] (1) Instruments and conditions
[0061] Shimadzu LC-MS / MS8050 high performance liquid chromatography triple quadrupole mass spectrometer;
[0062] Column: Alltima C 18 Chromatographic column (150mm×4.6mm, 5μm);
[0063] Mobile phase: 0.02mol / L ammonium acetate: acetonitrile (40:60);
[0064] Flow rate: 0.5ml / min;
[0065] Column temperature: 40°C;
[0066] Injection volume: 10μL;
[0067] Solvent: acetonitrile-water (50:50).
[0068] Mass spectrometry conditions: using electrospray ionization source (ESI), positive ion scanning mode;
[0069] The atomizing gas, drying gas and heating gas are nitrogen, and the collision gas is argon;
[0070] The atomizing gas flow rate is 3L / min;
[0071] The drying gas flow rate is 10.0L / min;
[0072] The heating gas flow rate is 10.0L / min;
[0073] The interface temperature is 300°C;
[0074] The temperat...
Embodiment 2
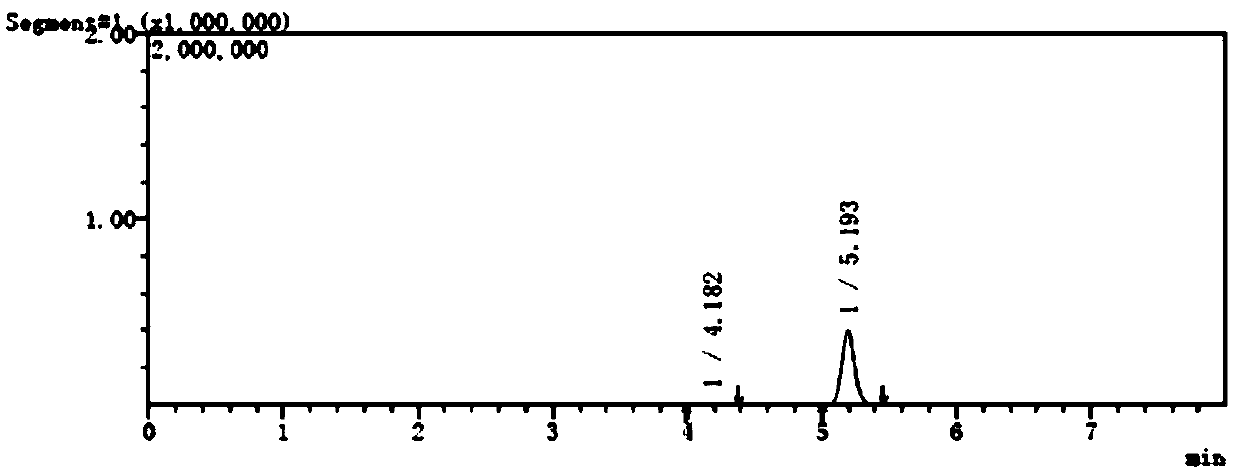
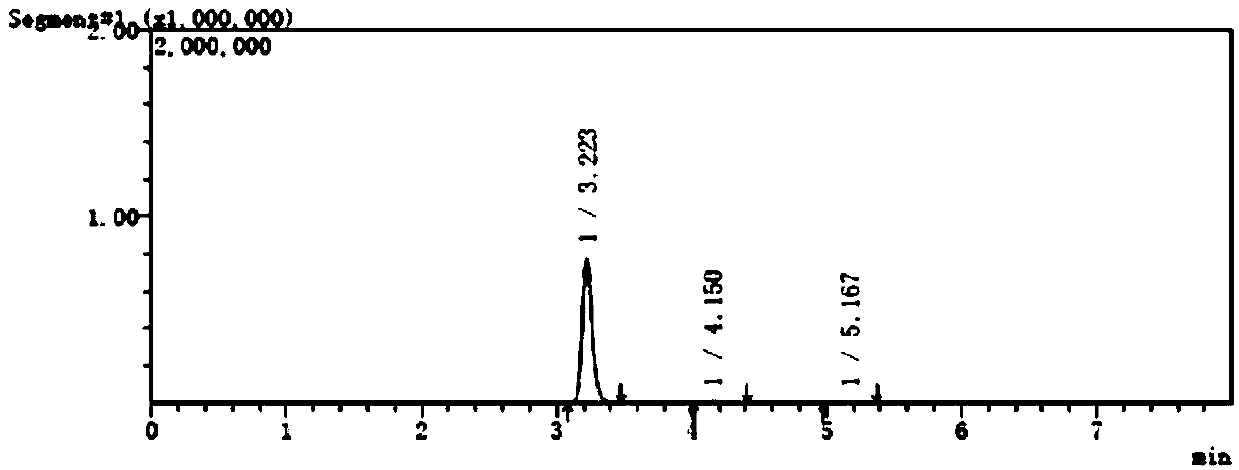
[0090] Example 2, Separation and determination method of potential genotoxic impurities in 5-difluoromethoxy-2-mercapto-1H-benzimidazole
[0091] (1) Instruments and conditions
[0092] Shimadzu Agilent 6410B Triple Quadrupole LC-MS high performance liquid chromatography-triple quadrupole tandem mass spectrometer;
[0093] Column: Alltima C 18 Chromatographic column (150mm×4.6mm, 5μm);
[0094] Mobile phase: 0.005Mol / L ammonium acetate aqueous solution (containing 0.1% formic acid): methanol (60:40)
[0095] Flow rate: 0.5ml / min;
[0096] Column temperature: 40°C;
[0097] Injection volume: 10μL;
[0099] Mass spectrometry conditions: ion source is electrospray ion source, positive ion scanning mode;
[0100] Gas Temp: 300℃;
[0101] Gas Flow: 10L / min;
[0102] Nebulizer: 30psi;
[0103] Capillary: 5500V.
[0104] MRM detection mode, the parameters are as follows:
[0105]
[0106]
[0107] (2) Experimental steps
[0108] Sample (5-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com