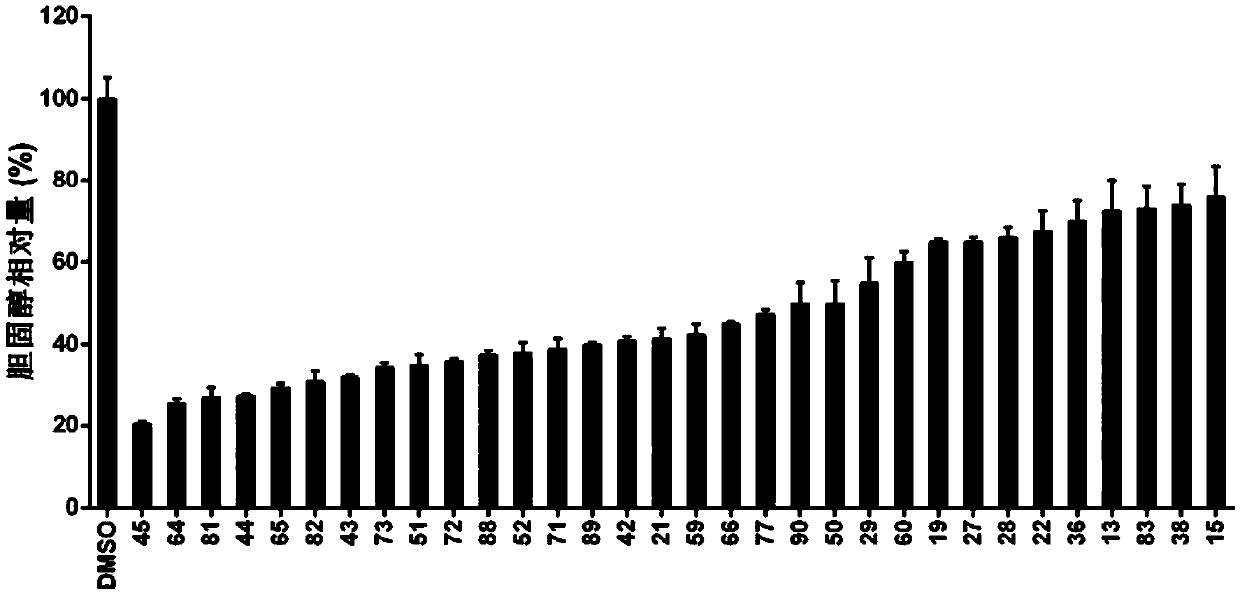
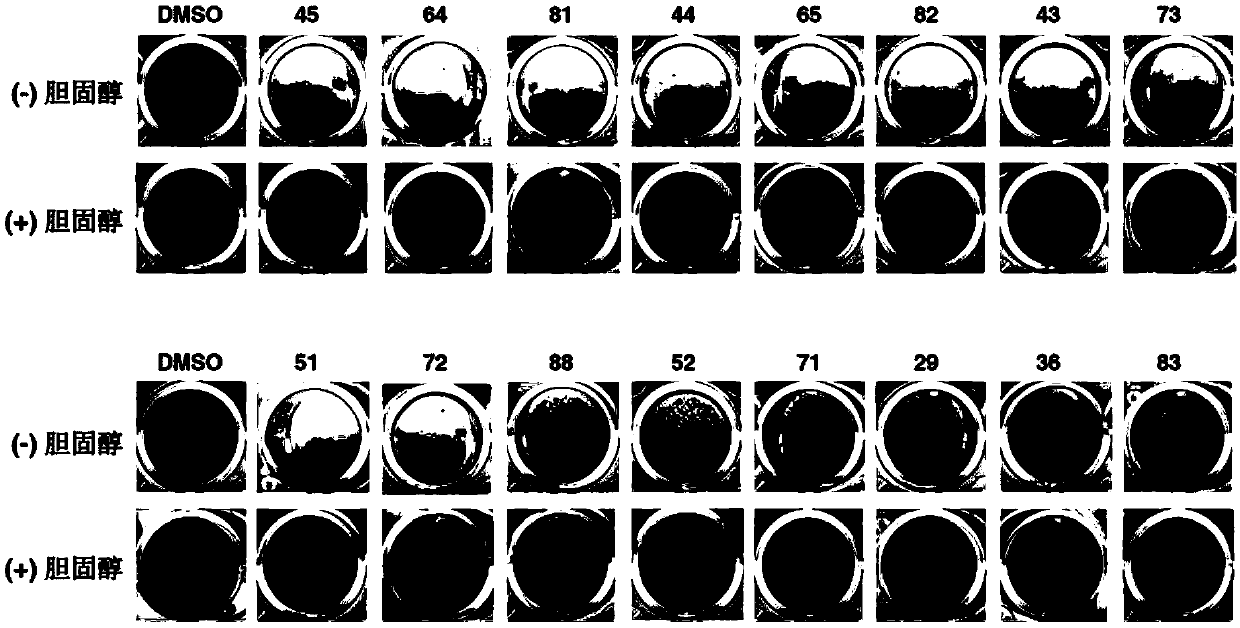
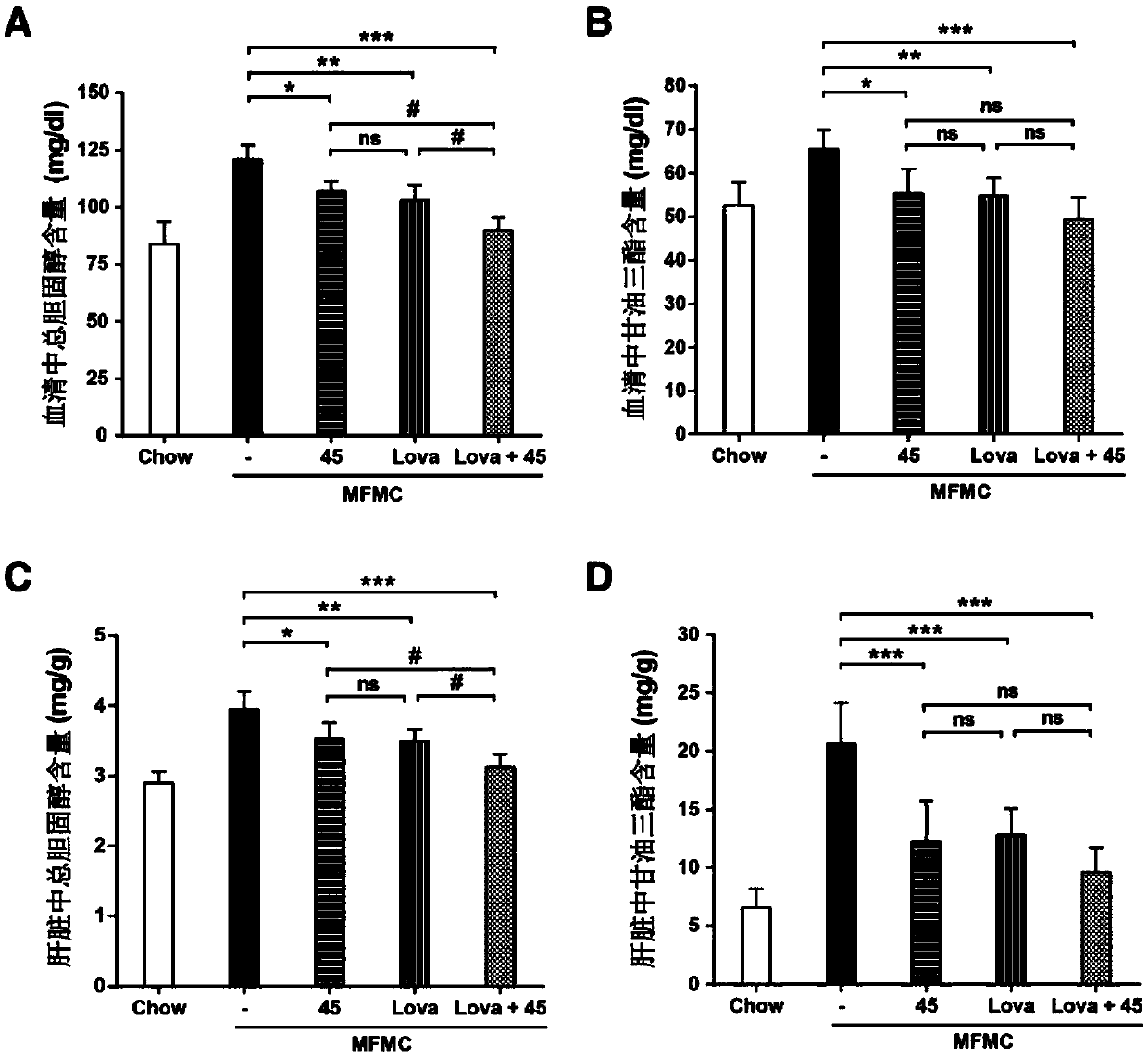
Cholic acid derivative and preparation method and application thereof
A kind of derivative, cholic acid technology, applied in the field of medicine and its preparation and application, can solve the problems of gastrointestinal discomfort, peripheral neuropathy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0262] Example 1 Preparation of Compounds 13 and 36
[0263] The compound Lithocholic acid (3.76g, 10mmol) was dissolved in anhydrous methanol (150mL), and SOCl was added dropwise at 0°C 2 (4.76g, 2.9mL, 40mmol), after the dropwise addition was completed, it was naturally warmed to room temperature and stirred for 5h. After the complete reaction was detected by TLC, the reaction solution was concentrated, then ethyl acetate (250 mL) was added to dissolve the residue, and the organic phase was successively washed with saturated NaHCO 3 solution (3×50mL), washed with saturated NaCl solution (3×50mL), anhydrous NaCl 2 SO 4 It was dried, concentrated, and purified by silica gel column chromatography (PE:EA=3:1) to obtain compound 1 (3.88 g, 99%) as a white solid. 1 H NMR (500MHz, CDCl 3 )δ3.67(s,3H),3.59-3.65(m,1H),2.30-2.40(m,1H),2.20-2.26(m,1H),1.93-1.99(m,1H),1.74-1.90( m,5H),1.63-1.73(m,2H),1.57-1.60(m,1H),1.48-1.54(m,1H),1.23-1.44(m,11H),1.02-1.17(m,5H), 0.92(s,3H),0.91...
Embodiment 2
[0281] Example 2 Preparation of Compounds 14, 37, 15, 38, 19 and 42
[0282] Compound 13 (56mg, 0.13mmol) was dissolved in anhydrous methanol (10mL). After complete dissolution, 4M NaOH solution (2mL) was added to the reaction system, and the system immediately became cloudy. After the addition was complete, it was stirred at room temperature for 24h. After the complete reaction was detected by TLC, the reaction solution was concentrated, and then H was added to the residue. 2 O (10mL), and then use 1M hydrochloric acid to adjust the pH of the solution to be about 3, a large amount of white solid is precipitated, the white solid is filtered out, and then the white solid is dissolved with anhydrous methanol, and anhydrous Na 2 SO 4 It was dried, concentrated, and purified by silica gel column chromatography (DCM:MeOH=20:1) to obtain compound 14 (52 mg, 95%) as a white solid. 1 H NMR (500MHz, DMSO-d 6 )δ11.94(brs,1H),5.36(d,J=2.7Hz,1H),4.50-4.56(m,1H),4.15-4.20(m,1H),3.58-3.6...
Embodiment 3
[0294] Example 3 Preparation of Compounds 20-22 and 43-45
[0295] Dissolve compound 13 (87mg, 0.2mmol) in anhydrous THF (12mL), slowly add CH 3 The THF solution of MgCl (1.0M, 2mL) was added dropwise, and reacted for about 0.5h after naturally rising to room temperature. After the complete reaction was detected by TLC, slowly add saturated NH 4 Cl solution until no bubbles were generated, the solid was filtered out with suction, the filtrate was extracted with ethyl acetate (3×50mL), the combined organic phases were washed with saturated NaCl solution (3×30mL), anhydrous NaCl 2 SO 4 It was dried, concentrated, and purified by silica gel column chromatography (PE:EA=1:1) to obtain compound 20 (69 mg, 80%) as a white solid. 1 H NMR (500MHz, DMSO-d 6 )δ5.35(d,J=2.3Hz,1H),4.51-4.54(m,1H),4.15-4.19(m,1H),4.02(s,1H),3.57-3.64(m,1H),2.96 -3.05(m,1H),1.89-1.95(m,1H),1.72-1.82(m,2H),1.57-1.66(m,2H),1.49-1.57(m,1H),1.36-1.46(m, 4H),1.27-1.36(m,4H),1.14-1.25(m,3H),1.02-1.07(m,4H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com