Method for synergistically catalyzing and oxidizing cycloalkane by porphyrin cobalt (II)/zinc (II) salt
A technology of synergistic catalysis, porphyrin cobalt, applied in chemical instruments and methods, oxidation reaction preparation, preparation of organic compounds, etc., to achieve the effects of being conducive to continuity, low selectivity, and inhibiting formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
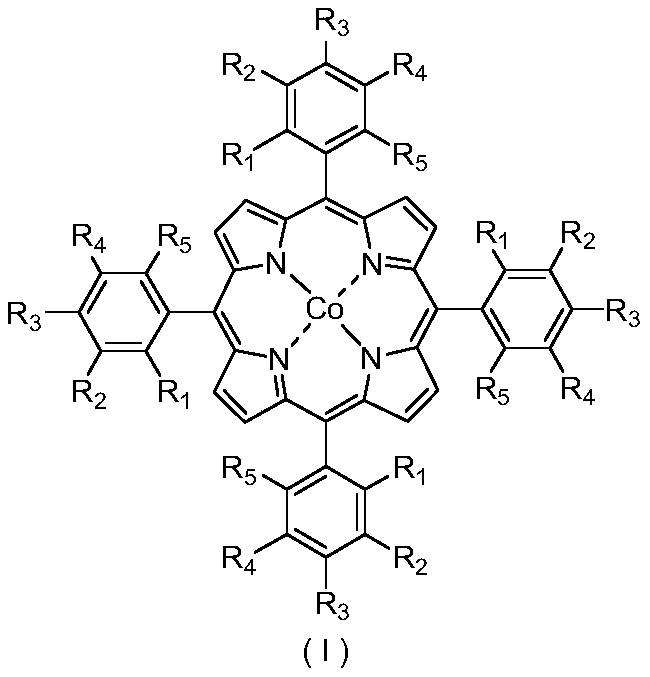
[0024] In a 100mL stainless steel autoclave with polytetrafluoroethylene liner, 0.0016g (0.0020mmol) 5,10,15,20- tetrakis (4-chlorophenyl) porphyrin cobalt (II) and 0.0367g (0.20 mmol) anhydrous zinc acetate dispersed in 16.8320 g (200 mmol) cyclohexane, seal the reaction vessel, stir and raise the temperature to 120° C., and feed oxygen to 1.0 MPa. At 120°C, 1.0MPa oxygen pressure, 800rpm stirring reaction for 8.0h. After completion of the reaction, cool to room temperature with ice water, add 1.3115g (5.00mmol) triphenylphosphine (PPh 3 ), stirred at room temperature for 30min to reduce the generated peroxide. Using acetone as solvent, the resulting reaction mixture was adjusted to 100 mL. Pipette 10mL of the resulting solution, and use toluene as an internal standard for gas chromatographic analysis; pipette 10mL of the resulting solution, and use benzoic acid as an internal standard for liquid chromatographic analysis. Cyclohexane conversion 5.39%, cyclohexanol selectiv...
Embodiment 2
[0026]In a 100mL stainless steel autoclave with polytetrafluoroethylene liner, 0.00016g (0.0002mmol) 5,10,15,20- tetrakis (4-chlorophenyl) porphyrin cobalt (II) and 0.0367g (0.20 mmol) anhydrous zinc acetate dispersed in 16.8320 g (200 mmol) cyclohexane, seal the reaction vessel, stir and raise the temperature to 120° C., and feed oxygen to 1.0 MPa. At 120°C, 1.0MPa oxygen pressure, 800rpm stirring reaction for 8.0h. After completion of the reaction, cool to room temperature with ice water, add 1.3115g (5.00mmol) triphenylphosphine (PPh 3 ), stirred at room temperature for 30min to reduce the generated peroxide. Using acetone as solvent, the resulting reaction mixture was adjusted to 100 mL. Pipette 10mL of the resulting solution, and use toluene as an internal standard for gas chromatographic analysis; pipette 10mL of the resulting solution, and use benzoic acid as an internal standard for liquid chromatographic analysis. Cyclohexane conversion 5.22%, cyclohexanol selectiv...
Embodiment 3
[0028] 0.1619g (0.20mmol) 5,10,15,20-tetrakis(4-chlorophenyl) porphyrin cobalt (II) and 0.0367g (0.20 mmol) anhydrous zinc acetate dispersed in 16.8320 g (200 mmol) cyclohexane, seal the reaction vessel, stir and raise the temperature to 120° C., and feed oxygen to 1.0 MPa. At 120°C, 1.0MPa oxygen pressure, 800rpm stirring reaction for 8.0h. After completion of the reaction, cool to room temperature with ice water, add 1.3115g (5.00mmol) triphenylphosphine (PPh 3 ), stirred at room temperature for 30min to reduce the generated peroxide. Using acetone as solvent, the resulting reaction mixture was adjusted to 100 mL. Pipette 10mL of the resulting solution, and use toluene as an internal standard for gas chromatographic analysis; pipette 10mL of the resulting solution, and use benzoic acid as an internal standard for liquid chromatographic analysis. Cyclohexane conversion 5.78%, cyclohexanol selectivity 53%, cyclohexanone selectivity 42%, cyclohexyl hydroperoxide selectivity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com