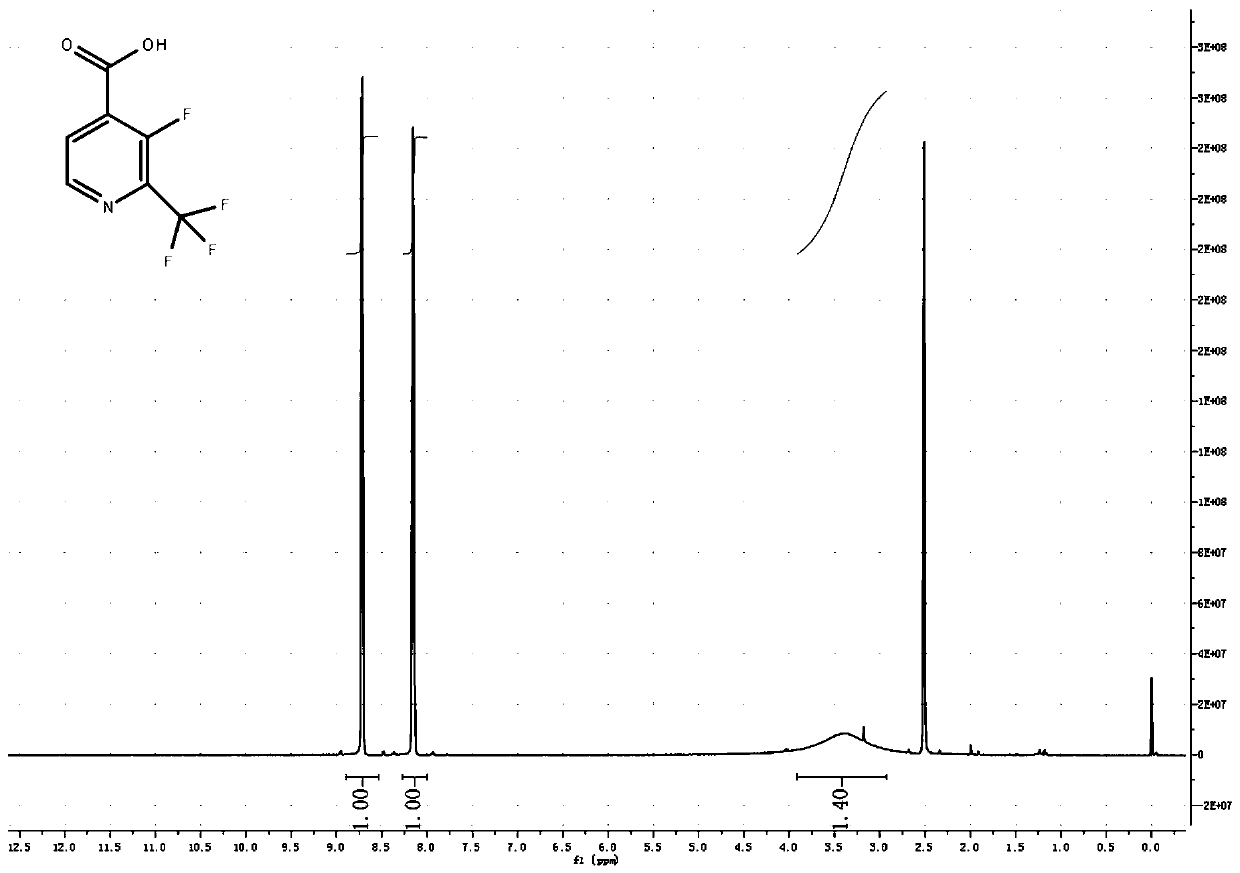
Synthesis method of 3-fluoro-2-trifluoromethyl isonicotinic acid
A technology of trifluoromethyl isofumine and a synthesis method, applied in the field of compound preparation, can solve the problems of high environmental cost, high price, no economic value and the like, and achieve the effects of high safety factor, convenient operation and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
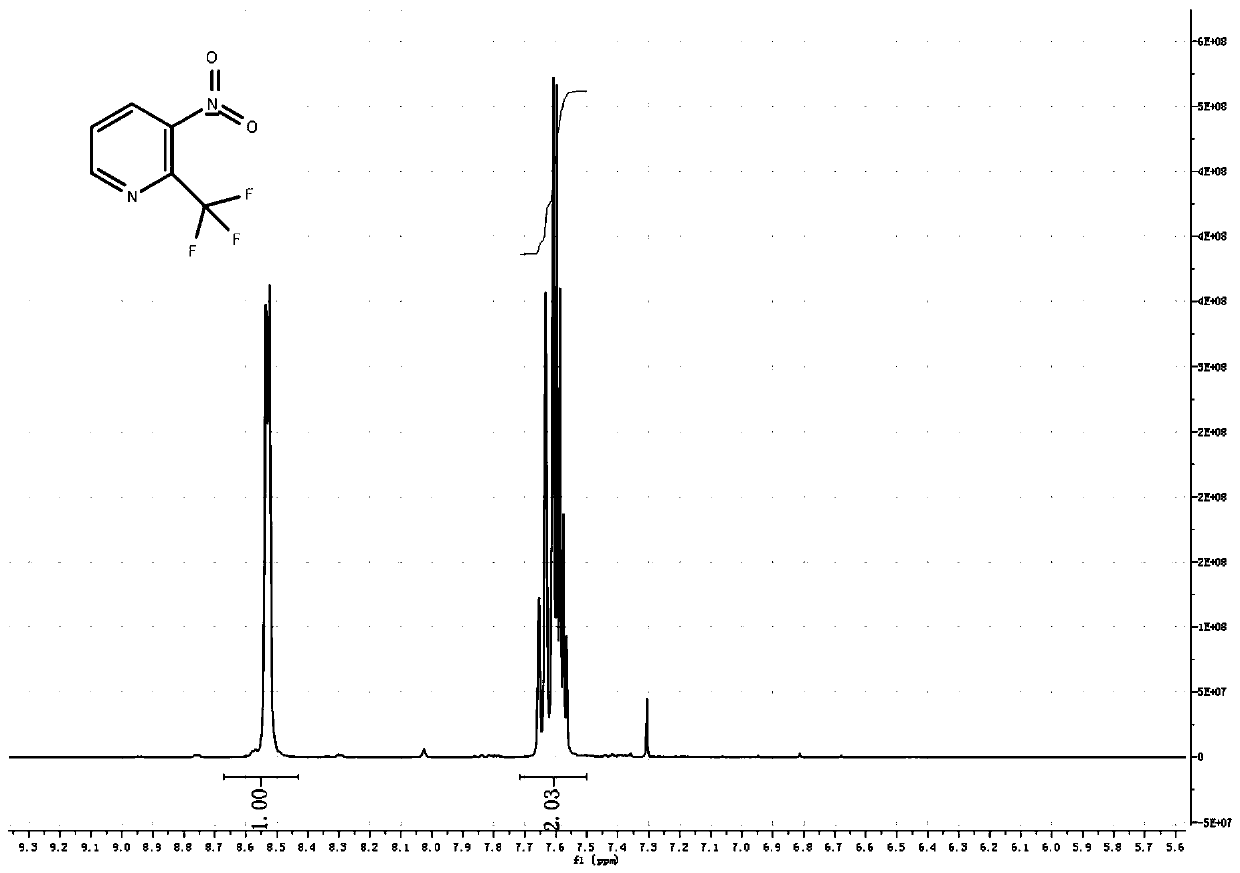
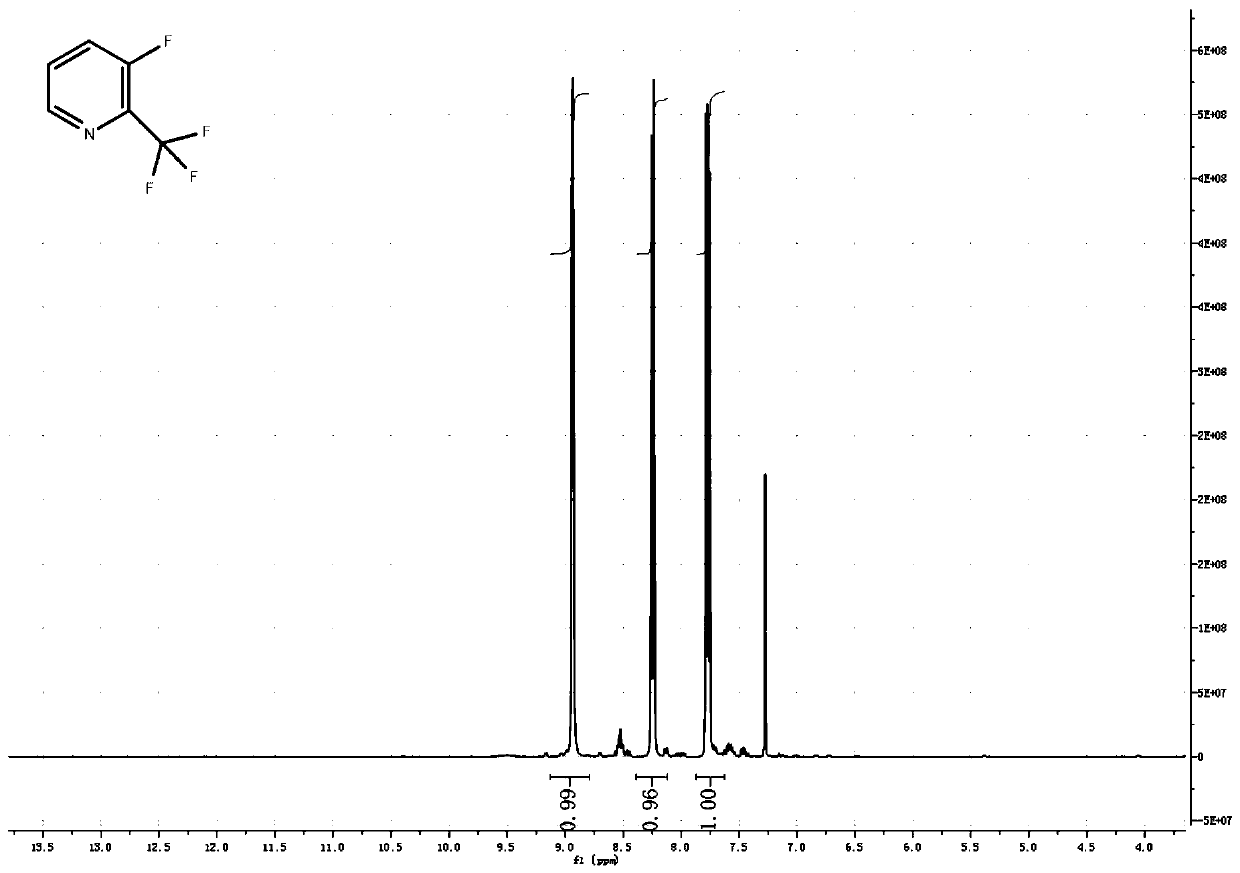
[0039] In a 500ml three-necked flask, equipped with a thermometer, nitrogen protection port, 2-chloro-3-nitropyridine 15.8g, 200ml DMF, nitrogen protection, add 1.9g cuprous iodide, 1.8g 1,10-phenanthroline, and raise the temperature to 100℃ , Add 21.3g of trifluoromethyltrimethylsilane dropwise, protected by nitrogen, after dropping, keep at 100℃, about 1 hour later, there is a process of rinsing the temperature to 105℃, then keep the temperature for 1 hour, TLC detection, raw material The reaction is complete. Pour the system into 1 liter of water, filter with suction, extract 3 times with 100ml methyl tert-butyl ether, wash with saturated sodium chloride, dry, and concentrate to obtain 25g of crude product, vacuum distillation, collect 5mmHg, 100~110℃ fraction Finished product, 12.1g, yield 64%.
Embodiment 2
[0041] In a 5-liter three-necked flask, equipped with a thermometer, nitrogen protection port, 2-chloro-3-nitropyridine 158g, 2LDMF, nitrogen protection, add 19g cuprous iodide, 18g 1,10-phenanthroline, heat up to 100℃, drop Add 213g of trifluoromethyltrimethylsilane, protect it with nitrogen, complete the dripping, and keep it at 100°C. After about 1 hour, the temperature is increased to 115°C in a flushing process, and the reaction is kept at the temperature for 2 hours. TLC detects that the raw material has reacted completely. Pour the system into 10 liters of water, filter with suction, extract 3 times with 1 liter of methyl tert-butyl ether, wash with saturated sodium chloride, dry, and concentrate to obtain 300 g of crude product. Distill under reduced pressure to collect 5mmHg, 100-110°C fraction The finished product was 103.6 g, and the yield was 54.2%.
Embodiment 3
[0043] In a 50-liter reaction flask, equipped with a thermometer, nitrogen protection port, 1.58kg 2-chloro-3-nitropyridine, 20LDMF, nitrogen protection, add 190g cuprous iodide, 180g 1,10-phenanthroline, and raise the temperature at 100℃, Add 2.2Kg of trifluoromethyltrimethylsilane dropwise, protected by nitrogen, and keep the temperature at 100°C. After about 1 hour, there will be a warming process, turn on the cooling mode, control the temperature not to exceed 110°C, and control it at 100~ The reaction was kept at 110°C for 2 hours, and TLC detected that the reaction of the raw materials was complete. Cool the system to room temperature, filter with suction, concentrate most of DMF, pour it into 10 liters of water, filter again with suction to remove a little copper compounds, extract 3 times with 5 liters of methyl tert-butyl ether, wash with saturated sodium chloride, and dry , Concentrated and dried to obtain 3kg of crude product, vacuum distillation, collect 5mmHg, 100 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com