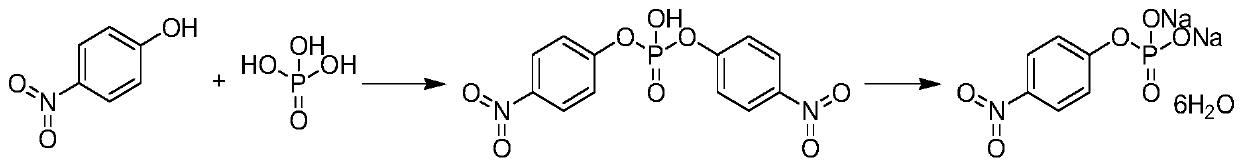
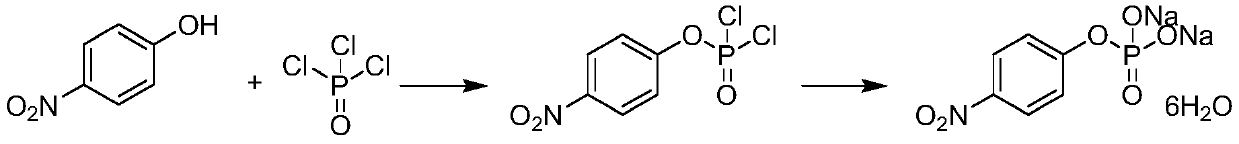
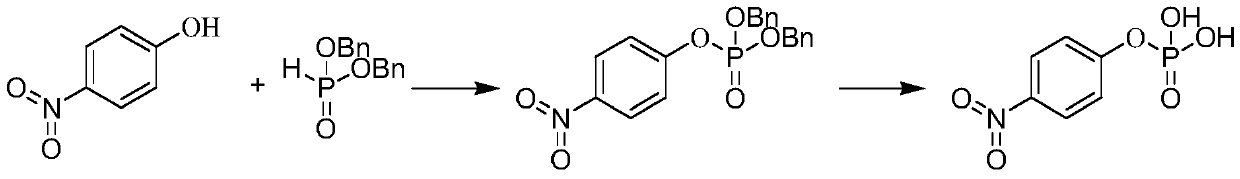
P-nitrophenyl phosphate disodium and preparation method thereof
A technology of disodium nitrophenyl phosphate and nitrophenyl phosphate, applied in the field of disodium p-nitrophenyl phosphate and its preparation, can solve the problems of unsuitable for industrial production, expensive dibenzyl phosphite, carbon tetrachloride Toxicity and other problems, to achieve good application prospects, reduce the difficulty of purification, the effect of thorough hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The preparation method of disodium p-nitrophenyl phosphate according to the embodiment of the present invention comprises the following steps:
[0037] Step 1: react p-nitrophenol with dialkyl chlorophosphate in the presence of alkali to obtain O, O-dialkyl p-nitrophenyl phosphate.
[0038] Its reaction formula is shown in following formula (1):
[0039]
[0040] Wherein, the dialkyl chlorophosphate is preferably selected from dimethyl chlorophosphate, diethyl chlorophosphate, diisopropyl chlorophosphate, or a mixture thereof. In other words, the aforementioned R is preferably methyl, ethyl, or propyl.
[0041] The reaction is preferably carried out at 0-25°C, and the reaction time is preferably 4-10 hours.
[0042] Wherein, the base is an inorganic base and / or an organic base, and the inorganic base is selected from sodium carbonate, potassium carbonate, sodium bicarbonate, potassium bicarbonate, sodium hydride, potassium hydride, or a mixture thereof, and the org...
Embodiment 1
[0063] (1) Synthesis of O, O-diethyl-p-nitrophenyl phosphate
[0064] Suspend p-nitrophenol (139 g, 1.0 mol) in dichloromethane and cool to . After quickly dropping triethylamine (121.4g, 1.2mol) into the reaction flask, continue to maintain 0°C and slowly add diethyl chlorophosphate (207g, 1.2mol) dropwise, and then naturally rise to room temperature after dropping, and continue to react for 4h , liquid phase monitoring p-nitrophenol is almost consumed, stop the reaction.
[0065] The reaction solution was washed with saturated sodium bicarbonate, 1N dilute hydrochloric acid and water respectively, and the organic phase was collected, dried with anhydrous sodium sulfate, filtered and concentrated to obtain 280 g of crude product.
[0066] Thereafter, vacuum distillation was carried out to obtain 260 g of O, O-diethyl-p-nitrophenyl phosphate, with a yield of 94.4%.
[0067] This intermediate is an inhibitor of cholinesterase, and appropriate protection measures should be tak...
Embodiment 2
[0085] (1) Synthesis of O, O-diethyl-p-nitrophenyl phosphate
[0086] It was prepared in the same manner as in Example 1 above, and its detailed description is omitted here.
[0087] (2) Synthesis of O, O-bis(trimethylsilyl)-p-nitrophenyl phosphate
[0088] O,O-diethyl-p-nitrophenyl phosphate (260 g, 0.95 mol) and acetonitrile were added to the reaction flask. After cooling down to 0° C. in an ice bath, TMSCl (1032.1 g, 9.5 mol) was added dropwise thereto, and after the drop was completed, it was naturally raised to room temperature, and the reaction was continued for 24 h, and the reaction was completed by monitoring by phosphospectrum. The reaction solution was concentrated to obtain oily O, O-bis(trimethylsilyl)-p-nitrophenyl phosphate.
[0089] (3) Synthesis of p-nitrophenylphosphoric acid
[0090] After cooling the O,O-bis(trimethylsilyl)-p-nitrophenyl phosphate obtained in the above (2) to 0°C, drop the mixture of methanol and water into the reaction flask while stirr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com