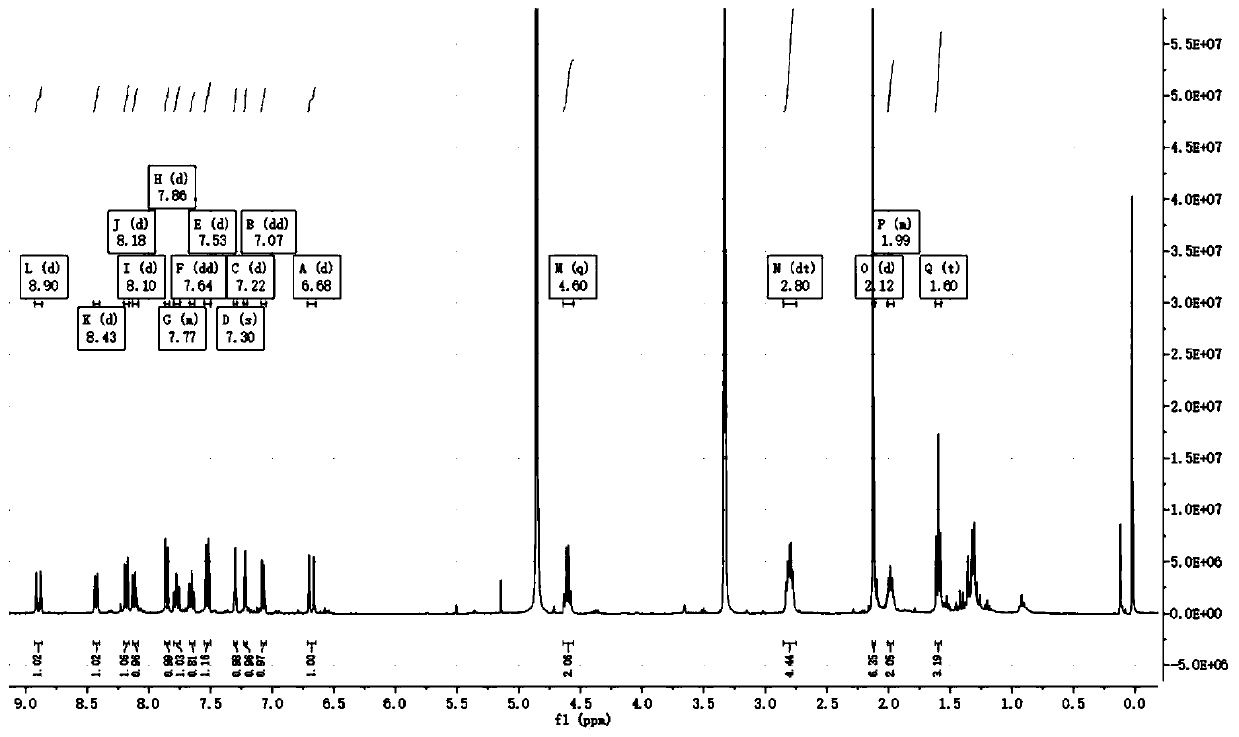
Near-infrared fluorescent molecular probe for detecting hydrogen sulfide and preparation method and application of near-infrared fluorescent molecular probe
A fluorescent molecular probe, near-infrared technology, applied in chemical instruments and methods, fluorescence/phosphorescence, material analysis by optical means, etc., can solve the problems of autofluorescence interference, difficult fluorescence, etc. Strong and responsive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of near-infrared fluorescent probes for detecting hydrogen sulfide:
[0037] Add a certain amount of compound 1 into a round bottom flask, slowly add N,N-dimethylformamide in ice bath and nitrogen protection, after cooling for 10min, start stirring, and slowly add in ice bath and nitrogen protection After tert-butyl nitrite was stirred for 5 minutes, trimethylsilyl azide was slowly added under the conditions of ice bath and oxygen protection. After stirring for 10 minutes, remove the ice bath, put it into an oil bath, stir and heat to 80 degrees Celsius. React for a period of time, wait for the solution to turn blue, cool the system, pour the reaction solution in the bottle into the separatory funnel, add distilled water, after fully mixing, add dichloromethane, continue to shake for 5 minutes, let the separatory funnel stand, wait After the solution was separated in the separatory funnel, the solution in the lower layer was evaporated to dryness by vacuum ...
Embodiment 2
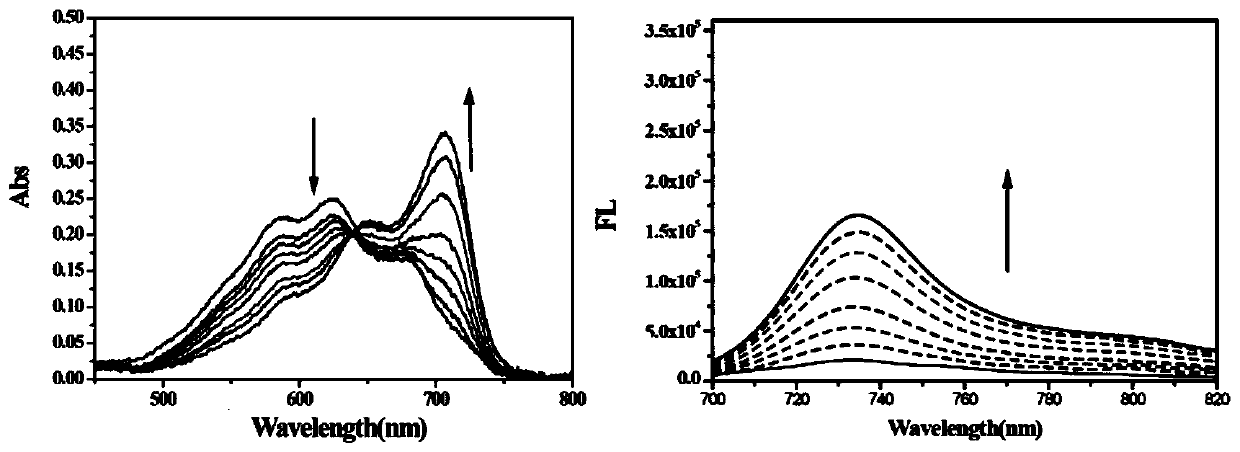
[0042] Near-infrared fluorescent probe and hydrogen sulfide in vitro response to ultraviolet absorption and fluorescence emission measurement experiments:
[0043] Weigh 4.7mg probe and dissolve it in 1mL DMSO to make 10mM probe stock solution. Weigh 5.6mg NaHS and dissolve it in 10mL water to make 10mM NaHS mother solution. Prepare eight 5mL centrifuge tubes, add 3mL of PBS buffer solution (pH=7.4) to each centrifuge tube in turn, and then add 3 μL of probe mother solution. At this time, the probe concentration in each centrifuge tube is 10 μM. After fully mixing, add 0, 6, 12, 18, 24, 30, 36, 42 μL of NaHS mother solution to each centrifuge tube respectively, and after reacting for 20 minutes, add them to the cuvette in order for UV absorption and fluorescence emission. Detection (excitation light wavelength is 690nm), the obtained data are processed by origin software figure 2 , indicating that the probe responds well to hydrogen sulfide in solution.
Embodiment 3
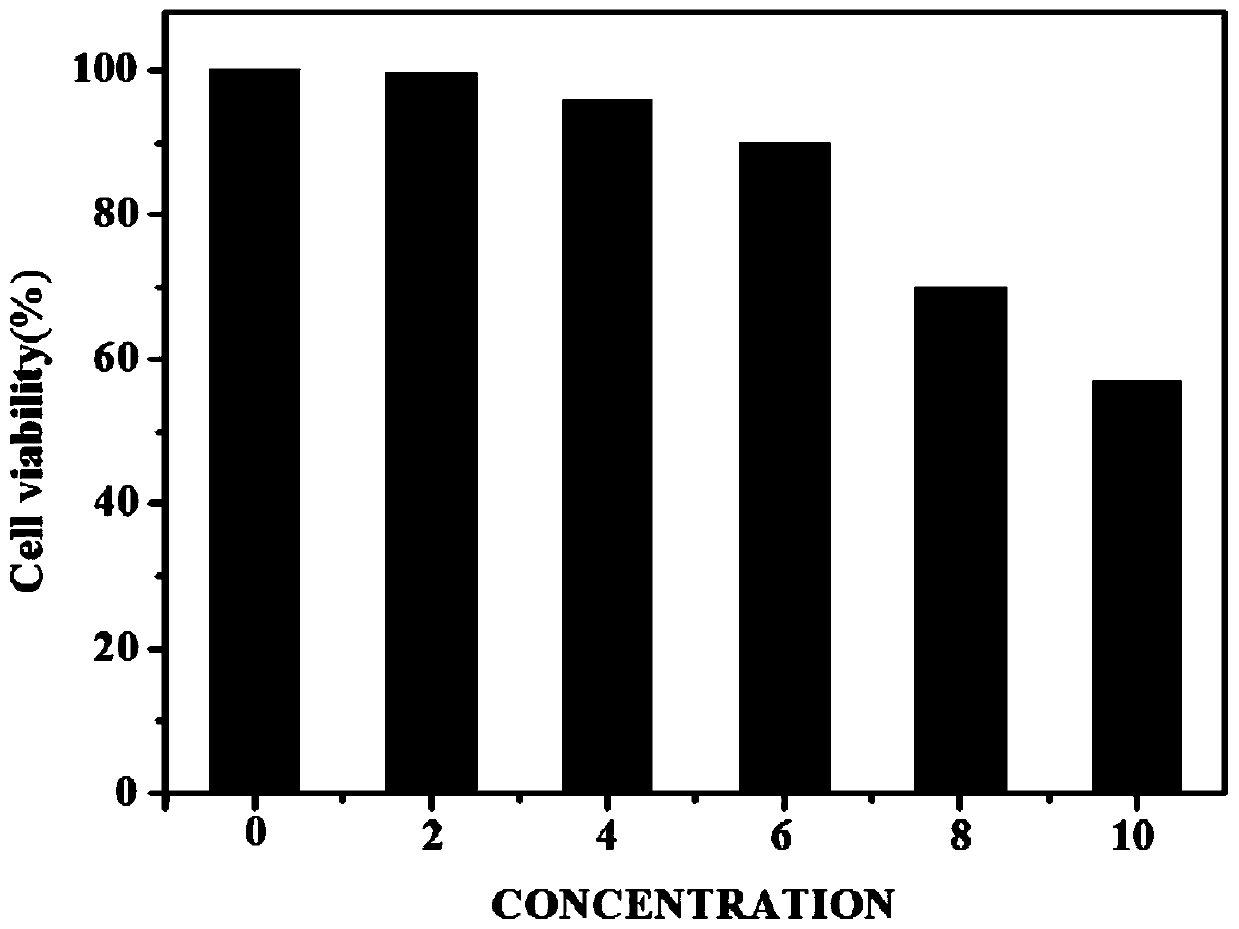
[0045] MTT biocompatibility experiment between near-infrared fluorescent probe and U87 cells:
[0046] PBS solution was added to the periphery of the 96-well plate, and the remaining inner wells were covered with U87 cells, and cultured for 12 hours. Weigh 4.7mg of the probe and dissolve it in 5mL of biological DMSO solution to prepare a 2mM biological probe stock solution. Prepare 5mg / mL MTT solution. Prepare six 5mL centrifuge tubes, add 2mL medium to each, and add 0, 2, 4, 6, 8, 10 μL of biological probe master solution to the centrifuge tubes in sequence. Pipette 190 μL of the solution in the 6 centrifuge tubes and add the same concentration to the 6 rows of wells in sequence and incubate for 24 hours, then add 10 μL of MTT solution to each well. Continue to incubate for 3 hours, discard the medium in the liquid, and add 100 μL DMSO solution to each well. After fully dissolving, carry out ultraviolet absorption detection (detection wavelength is 490nm). Data processing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com