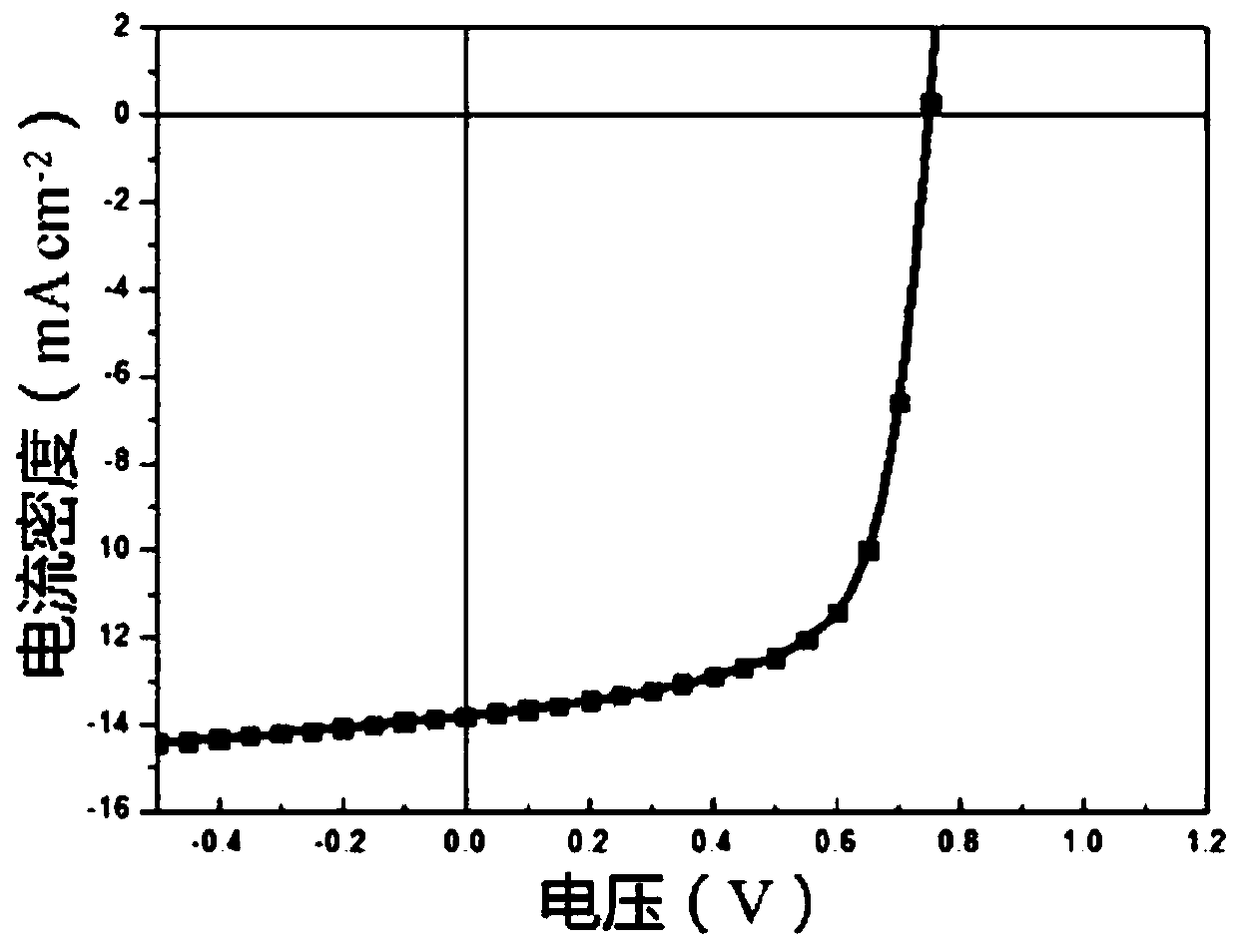
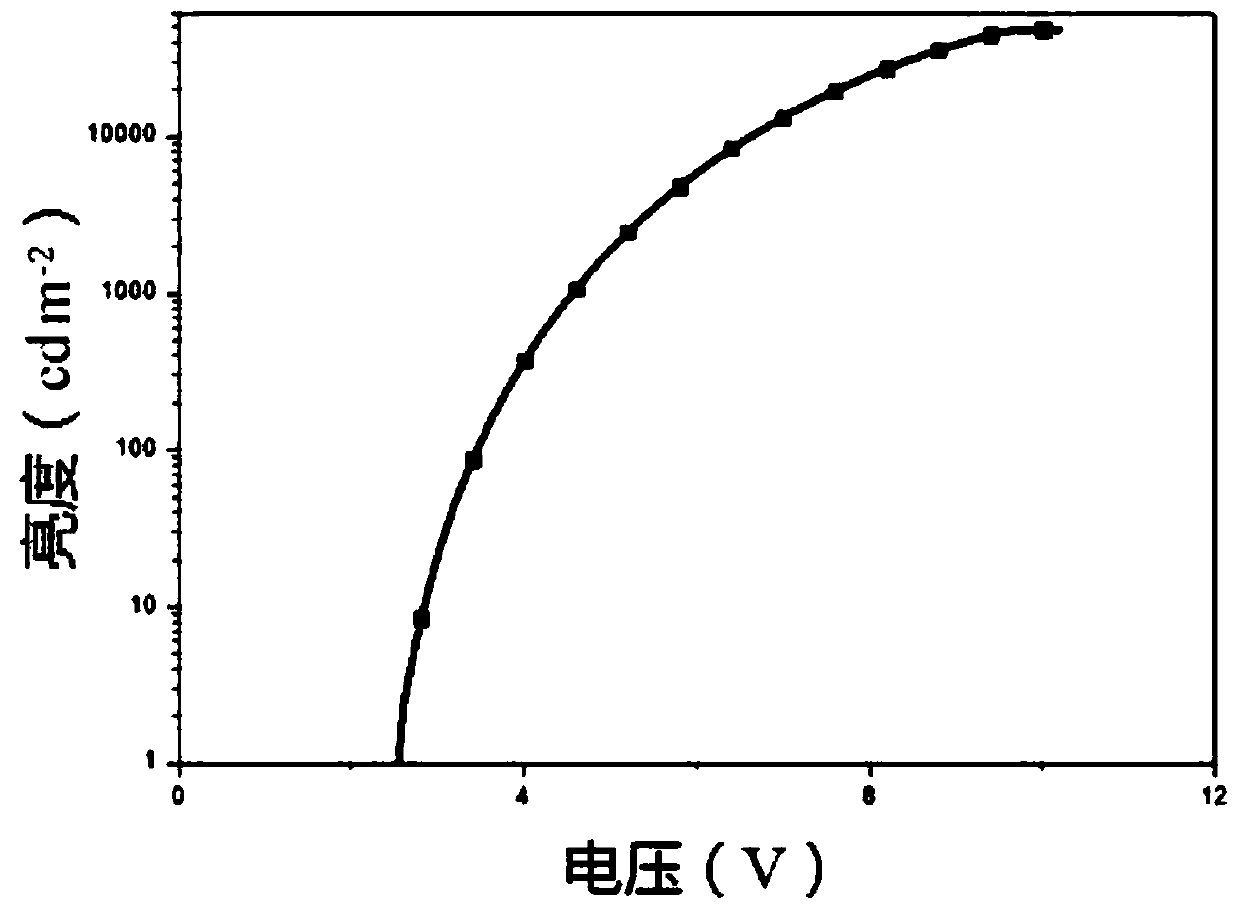
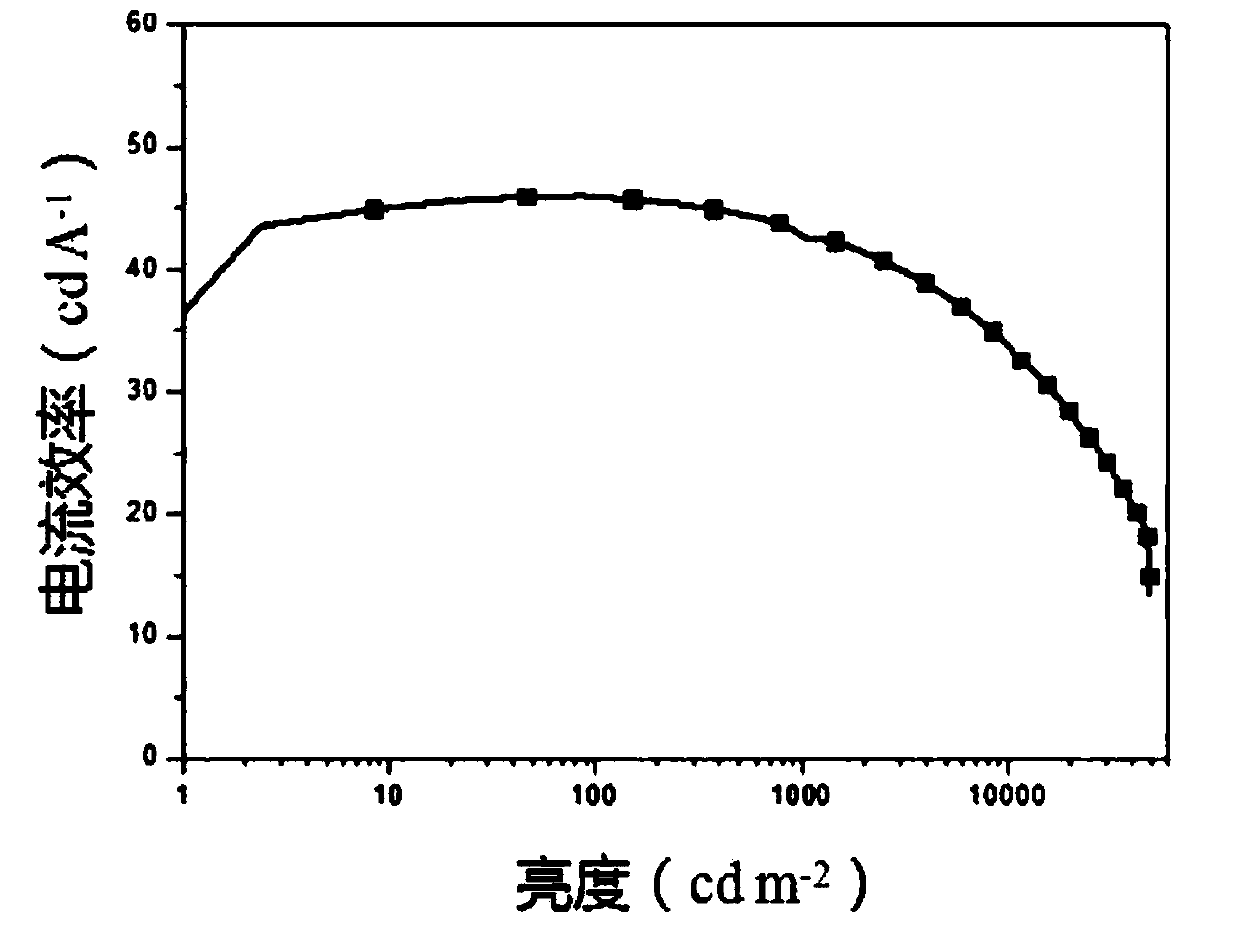
Hyperbranched conjugated polyelectrolyte based on tripolyindole and preparation method and use thereof
A technology of conjugated polyelectrolyte and tripolybenzazole, which is applied in the direction of circuits, photovoltaic power generation, electrical components, etc., can solve the problems of low device efficiency, poor film formation, difficult orthogonal solvent solution processing and preparation, etc., and achieve good transparency The effect of light efficiency and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The present invention also provides a method for preparing a hyperbranched conjugated polyelectrolyte based on tripolybenzazole, comprising the following steps:
[0062] Step 1. In the presence of NaH, the compound of formula (V) is subjected to an electrophilic substitution reaction to form a small molecule compound of formula (VI);
[0063]
[0064] M is the following structure:
[0065]
[0066] The structure of R is shown in formulas (II), (III) and (IV):
[0067]
[0068] Step 2-1. In the presence of a catalyst, the compound of formula (VI) undergoes a Suzuki coupling reaction to form a hyperbranched conjugated polyelectrolyte of formula (I);
[0069]
[0070] Or step 2-2, in the presence of a catalyst, the compound of formula (VI) undergoes a Stille coupling reaction, thereby forming a hyperbranched conjugated polyelectrolyte of formula (I);
[0071]
[0072] Or step 2-3, in the presence of a catalyst, the compound of formula (VI) undergoes a dire...
Embodiment 1
[0082] A tripolybenzazole-based hyperbranched conjugated polyelectrolyte whose structure is TAT-H-P, the synthetic route is as follows:
[0083]
[0084] (1) Synthesis of the intermediate with the chemical structure a: under the protection of argon, add 2-indolinone (3 g, 22.5 mmol) and phosphorus oxychloride (10 mL) into a 100 mL two-neck flask, and heat to 100 ° C for 12 h. After the reaction, cool to room temperature, add ice water and stir, add sodium hydroxide aqueous solution to neutralize the acidity of the system until the pH is neutral. Filtrate, dissolve the filter residue in acetone, dry with anhydrous sodium sulfate, spin dry after filtration, and separate by column chromatography, the eluent is petroleum ether / ethyl acetate (5:1), and recrystallized in acetone to obtain 0.75g slightly yellow crystals. Rate 29.0%. 1H NMR (400MHz, DMSO) δ11.87(s, 3H), 8.68(d, J=7.5Hz, 3H), 7.73(d, J=7.8Hz, 3H), 7.36(dt, J=22.3, 7.2Hz ,6H).
[0085] (2) Synthesis of intermediat...
Embodiment 2
[0090] A tripolybenzazole-based hyperbranched conjugated polyelectrolyte whose structure is TAT-T-P, the synthetic route is as follows:
[0091]
[0092] Wherein, the synthesis of intermediates a, b, and c is the same as in Example 1.
[0093] Synthesis of TAT-T-P: Under argon protection, compound c (300mg, 0.28mmol), thiophene-2,5-diboronic acid dipinacol ester (72.1mg, 0.42mmol), tetrakis(triphenyl Phosphine) palladium (9.9mg, 0.0085mmol), sodium carbonate (226.5mg, 2.14mmol), 12mL DMF, 3mL water, react at 90°C for 12h. The capping reaction of thiophene-2-boronic acid pinacol ester took 12 hours, and the capping reaction of 2-bromothiophene took 12 hours. After the reaction, cool to room temperature, settle with acetone, filter, and dialyze in water (the molecular weight cut-off of the dialysis bag is 3500). Obtained 249.1mg black bright powder, yield 93.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com